Assessing 'no evidence of disease activity' status in patients with relapsing-remitting multiple sclerosis: a long-term follow-up
- PMID: 37609659
- PMCID: PMC10440375
- DOI: 10.3389/fneur.2023.1187851
Assessing 'no evidence of disease activity' status in patients with relapsing-remitting multiple sclerosis: a long-term follow-up
Abstract
Introduction: Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease of the CNS with an autoimmune pathogenesis. Over the years, numerous disease-modifying therapies (DMTs) have proven effective in disease control; to date, there is a need to identify a personalized treatment effective in ensuring disease-free status or no evidence of disease activity (NEDA).
Objective: identify clinical, demographic and treatment approach characteristics that affect the maintenance of NEDA-3 and the occurrence of clinical relapses during a 6-years follow-up.
Materials and method: a retrospective study was conducted on a cohort of MS patients followed up with six-year period. All participants were treated with first- or second-line MS drugs.Clinical relapse, NEDA-3 at 6 years and sustained EDSS were assessed as disease activity outcomes. Patients with follow-up of less than 6 years and insufficient clinical and radiological data were excluded from the study.
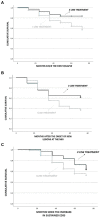
Results: Two-hundred-eighty naive patients (mean age was 49.8 years, SD ± 11.35 years, 23-76, F/M 182/98), with MS were followed up for 6 years.The mean age at diagnosis was 34.3 years (SD ±11.5, 14-62 years), the mean EDSS score at the onset was 1.9 (±1.3), 76.8% of patients had an EDSS below or equal to 2.5 at diagnosis.In the cohort 37 (13.2%) directly received second-line treatment, 243 (86.8%) received first-line drugs.The analysis showed that second-line treatment from beginning had a protective effect for the achievement of NEDA-3 (p = 0.029), on the prevention of clinical relapse (p = 0.018) and on number of relapses (p = 0.010); this finding was confirmed by logistic regression analysis (p = 0.04) and Kaplan-Meier analysis (p = 0.034).
Conclusion: The results of this study demonstrate the efficacy of targeted and early intervention so as to act in the right time window, ensuring a favorable outcome in both clinical and radiological terms; this could be decisive in reducing clinical relapse, disease progression and related disability. Therefore, prescribing highly effective drug in the early stages of the disease represents a leading strategy with the most favorable cost-benefit ratio.
Keywords: DMT; EDSS; MRI; NEDA; multiple sclerosis; relapse; second-line treatment.
Copyright © 2023 Zilli, Scribani Rossi, Di Stadio, Fratino, Giuliani, Annecca, Russo, Di Piero and Altieri.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Weinstock-Guttman B, Medin J, Khan N, Korn JR, Lathi E, Silversteen J, et al. . Assessing 'No evidence of disease Activity' status in patients with relapsing-remitting multiple sclerosis receiving Fingolimod in routine clinical practice: a retrospective analysis of the multiple sclerosis clinical and magnetic resonance imaging outcomes in the USA (MS-MRIUS) study. CNS Drugs. (2018) 32:84. doi: 10.1007/s40263-017-0482-4, PMID: - DOI - PMC - PubMed
-
- Giovannoni G, Cook S, Rammohan K, Rieckmann P, Sorensen PS, Vermersch P, et al. . Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. (2011) 10:329–37. doi: 10.1016/S1474-4422(11)70023-0, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources