Abnormal Lymphatic Sphingosine-1-Phosphate Signaling Aggravates Lymphatic Dysfunction and Tissue Inflammation
- PMID: 37609838
- PMCID: PMC10592179
- DOI: 10.1161/CIRCULATIONAHA.123.064181
Abnormal Lymphatic Sphingosine-1-Phosphate Signaling Aggravates Lymphatic Dysfunction and Tissue Inflammation
Abstract
Background: Lymphedema is a global health problem with no effective drug treatment. Enhanced T-cell immunity and abnormal lymphatic endothelial cell (LEC) signaling are promising therapeutic targets for this condition. Sphingosine-1-phosphate (S1P) mediates a key signaling pathway required for normal LEC function, and altered S1P signaling in LECs could lead to lymphatic disease and pathogenic T-cell activation. Characterizing this biology is relevant for developing much needed therapies.
Methods: Human and mouse lymphedema was studied. Lymphedema was induced in mice by surgically ligating the tail lymphatics. Lymphedematous dermal tissue was assessed for S1P signaling. To verify the role of altered S1P signaling effects in lymphatic cells, LEC-specific S1pr1-deficient (S1pr1LECKO) mice were generated. Disease progression was quantified by tail-volumetric and -histopathologic measurements over time. LECs from mice and humans, with S1P signaling inhibition, were then cocultured with CD4 T cells, followed by an analysis of CD4 T-cell activation and pathway signaling. Last, animals were treated with a monoclonal antibody specific to P-selectin to assess its efficacy in reducing lymphedema and T-cell activation.
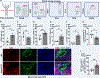
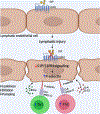
Results: Human and experimental lymphedema tissues exhibited decreased LEC S1P signaling through S1P receptor 1 (S1PR1). LEC S1pr1 loss-of-function exacerbated lymphatic vascular insufficiency, tail swelling, and increased CD4 T-cell infiltration in mouse lymphedema. LECs, isolated from S1pr1LECKO mice and cocultured with CD4 T cells, resulted in augmented lymphocyte differentiation. Inhibiting S1PR1 signaling in human dermal LECs promoted T-helper type 1 and 2 (Th1 and Th2) cell differentiation through direct cell contact with lymphocytes. Human dermal LECs with dampened S1P signaling exhibited enhanced P-selectin, an important cell adhesion molecule expressed on activated vascular cells. In vitro, P-selectin blockade reduced the activation and differentiation of Th cells cocultured with shS1PR1-treated human dermal LECs. P-selectin-directed antibody treatment improved tail swelling and reduced Th1/Th2 immune responses in mouse lymphedema.
Conclusions: This study suggests that reduction of the LEC S1P signaling aggravates lymphedema by enhancing LEC adhesion and amplifying pathogenic CD4 T-cell responses. P-selectin inhibitors are suggested as a possible treatment for this pervasive condition.
Keywords: P-selectin; T lymphocytes; lymphatic endothelial cells; lymphedema; sphingosine-1-phosphate receptors 1.
Conflict of interest statement
Figures






Update of
-
Abnormal lymphatic S1P signaling aggravates lymphatic dysfunction and tissue inflammation.medRxiv [Preprint]. 2023 Jun 12:2023.06.08.23291175. doi: 10.1101/2023.06.08.23291175. medRxiv. 2023. Update in: Circulation. 2023 Oct 17;148(16):1231-1249. doi: 10.1161/CIRCULATIONAHA.123.064181. PMID: 37398237 Free PMC article. Updated. Preprint.
Comment in
-
Sphingolipid Signaling Takes Center Stage in Regulation of Lymphatic-Immune Crosstalk in Lymphedema.Circulation. 2023 Oct 17;148(16):1250-1253. doi: 10.1161/CIRCULATIONAHA.123.066578. Epub 2023 Oct 16. Circulation. 2023. PMID: 37844150 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

