A novel device for atrial septal defect occlusion (GORE CARDIOFORM)
- PMID: 37609882
- PMCID: PMC10654763
- DOI: 10.4244/EIJ-D-23-00378
A novel device for atrial septal defect occlusion (GORE CARDIOFORM)
Abstract
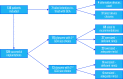
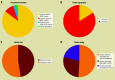
The GORE CARDIOFORM atrial septal defect (ASD) Occluder (GCA) is composed of a platinum-filled nitinol wire frame covered with expanded polytetrafluoroethylene, making it softer and more conformable compared with nitinol mesh devices. After the ASSURED clinical study confirmed the efficacy and safety of the device, it received U.S. Food and Drug Administration approval and a European conformity mark. Our aim was to understand the learning curve implicated in using the GCA for ASD closure in paediatric and adult patients as well as to study the early outcomes. To this end, a review of ASD device closures with GCA in 4 UK centres was conducted between January 2020 and January 2023. Implantation success was the primary outcome; the secondary outcomes were serious adverse events, including new onset arrhythmia. In all, 135 patients were included, and 128 (95%) had successful ASD device closure with GCA. The median patient age was 49 years, the median defect size was 18 mm, and the median device size was 37 mm. The median follow-up time was 6 months (interquartile range 1-14). One device embolisation occurred, and 15 patients (12% of GCA implantations) developed new onset arrhythmia - this was not related to patient age, defect diameter or device oversizing but was positively associated with device size. With growing experience using GCA, the device can be applied to a wide variety of ASD sizes and morphologies. Given the number of successful implantations with an absence of aortic erosion, as well as the ability to perforate through the device should procedures be required in the left atrium, the GCA device is an important addition for interventionists who close atrial septal defects.
Conflict of interest statement
The authors have no conflicts of interests to declare.
Figures


References
-
- Confident closure that is clinically proven for a wider range of ASDs. 2023. Jul 25, https://www.goremedical.com/products/cardioform/asd-occluder.
-
- Sommer RJ, Love BA, Paolillo JA, Gray RG, Goldstein BH, Morgan GJ, Gillespie MJ ASSURED Investigators. ASSURED clinical study: New GORE® CARDIOFORM ASD occluder for transcatheter closure of atrial septal defect. Catheter Cardiovasc Interv. 2020;95:1285–95. - PubMed
-
- Amanullah MM, Siddiqui MT, Khan MZ, Atiq MA. Surgical rescue of embolized amplatzer devices. J Card Surg. 2011;26:254–8. - PubMed
-
- Thanopoulos BVD, Soendergaard L, Ngugen HL, Marasini M, Giannopoulos A, Bompotis GC, Thonghong T, Krishnamoorthy KM, Placid S, Deleanou D, Toutouzas KP. International experience with the use of Cocoon septal occluder for closure of atrial septal defects. Hellenic J Cardiol. 2021;62:206–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

