Bifidobacterium infantis utilizes N-acetylglucosamine-containing human milk oligosaccharides as a nitrogen source
- PMID: 37609905
- PMCID: PMC10448974
- DOI: 10.1080/19490976.2023.2244721
Bifidobacterium infantis utilizes N-acetylglucosamine-containing human milk oligosaccharides as a nitrogen source
Abstract
Bifidobacterium longum subsp. infantis (B. infantis) utilizes oligosaccharides secreted in human milk as a carbohydrate source. These human milk oligosaccharides (HMOs) integrate the nitrogenous residue N-acetylglucosamine (NAG), although HMO nitrogen utilization has not been described to date. Herein, we characterize the B. infantis nitrogen utilization phenotype on two NAG-containing HMO species, LNT and LNnT. This was characterized through in vitro growth kinetics, incorporation of isotopically labeled NAG nitrogen into the proteome, as well as modulation of intracellular 2-oxoglutarate levels while utilizing HMO nitrogen. Further support is provided by comparative transcriptomics and proteomics that identified global regulatory networks deployed during HMO nitrogen utilization. The aggregate data demonstrate that B. infantis strains utilize HMO nitrogen with the potential to significantly impact fundamental and clinical studies, as well as enable applications.
Keywords: 2-oxoglutarate; bifidobacteria; human milk oligosaccharides; microbiota; nitrogen metabolism.
Conflict of interest statement
No potential conflict of interest was reported by the authors.
Figures

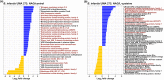

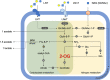
Similar articles
-
Human Milk Oligosaccharide Utilization in Intestinal Bifidobacteria Is Governed by Global Transcriptional Regulator NagR.mSystems. 2022 Oct 26;7(5):e0034322. doi: 10.1128/msystems.00343-22. Epub 2022 Sep 12. mSystems. 2022. PMID: 36094076 Free PMC article.
-
Broad conservation of milk utilization genes in Bifidobacterium longum subsp. infantis as revealed by comparative genomic hybridization.Appl Environ Microbiol. 2010 Nov;76(22):7373-81. doi: 10.1128/AEM.00675-10. Epub 2010 Aug 27. Appl Environ Microbiol. 2010. PMID: 20802066 Free PMC article.
-
Galacto- and Fructo-oligosaccharides Utilized for Growth by Cocultures of Bifidobacterial Species Characteristic of the Infant Gut.Appl Environ Microbiol. 2020 May 19;86(11):e00214-20. doi: 10.1128/AEM.00214-20. Print 2020 May 19. Appl Environ Microbiol. 2020. PMID: 32220841 Free PMC article.
-
Probiotics in the New Era of Human Milk Oligosaccharides (HMOs): HMO Utilization and Beneficial Effects of Bifidobacterium longum subsp. infantis M-63 on Infant Health.Microorganisms. 2024 May 17;12(5):1014. doi: 10.3390/microorganisms12051014. Microorganisms. 2024. PMID: 38792843 Free PMC article. Review.
-
Nursing our microbiota: molecular linkages between bifidobacteria and milk oligosaccharides.Trends Microbiol. 2010 Jul;18(7):298-307. doi: 10.1016/j.tim.2010.03.008. Epub 2010 Apr 19. Trends Microbiol. 2010. PMID: 20409714 Free PMC article. Review.
Cited by
-
Cross-feeding of bifidobacteria promotes intestinal homeostasis: a lifelong perspective on the host health.NPJ Biofilms Microbiomes. 2024 Jun 19;10(1):47. doi: 10.1038/s41522-024-00524-6. NPJ Biofilms Microbiomes. 2024. PMID: 38898089 Free PMC article. Review.
-
The neonatal gut microbiome in health and disease.Gut Microbes. 2025 Dec;17(1):2457499. doi: 10.1080/19490976.2025.2457499. Epub 2025 Jan 27. Gut Microbes. 2025. PMID: 39868670 Free PMC article. No abstract available.
References
-
- Sela DA; Chapman J; Adeuya A; Kim JH; Chen F; Whitehead TR; Lapidus A; Rokhsar DS; Lebrilla CB; German JB; Price NP; Richardson PM; Mills DA. The Genome Sequence of Bifidobacterium Longum Subsp. Infantis Reveals Adaptations for Milk Utilization within the Infant Microbiome. Proc Natl Acad Sci U S A. 2008;105(48):18964–18969. doi:10.1073/pnas.0809584105. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
