Negative regulation of biofilm formation by nitric oxide sensing proteins
- PMID: 37610010
- PMCID: PMC10625800
- DOI: 10.1042/BST20220845
Negative regulation of biofilm formation by nitric oxide sensing proteins
Abstract
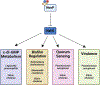
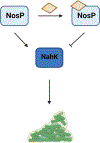
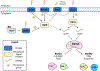
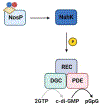
Biofilm-based infections pose a serious threat to public health. Biofilms are surface-attached communities of microorganisms, most commonly bacteria and yeast, residing in an extracellular polymeric substance (EPS). The EPS is composed of several secreted biomolecules that shield the microorganisms from harsh environmental stressors and promote antibiotic resistance. Due to the increasing prominence of multidrug-resistant microorganisms and a decreased development of bactericidal agents in clinical production, there is an increasing need to discover alternative targets and treatment regimens for biofilm-based infections. One promising strategy to combat antibiotic resistance in biofilm-forming bacteria is to trigger biofilm dispersal, which is a natural part of the bacterial biofilm life cycle. One signal for biofilm dispersal is the diatomic gas nitric oxide (NO). Low intracellular levels of NO have been well documented to rapidly disperse biofilm macrostructures and are sensed by a widely conserved NO-sensory protein, NosP, in many pathogenic bacteria. When bound to heme and ligated to NO, NosP inhibits the autophosphorylation of NosP's associated histidine kinase, NahK, reducing overall biofilm formation. This reduction in biofilm formation is regulated by the decrease in secondary metabolite bis-(3'-5')-cyclic dimeric guanosine monophosphate (c-di-GMP). The NosP/NahK signaling pathway is also associated with other major regulatory systems in the maturation of bacterial biofilms, including virulence and quorum sensing. In this review, we will focus on recent discoveries investigating NosP, NahK and NO-mediated biofilm dispersal in pathogenic bacteria.
Keywords: NosP; biofilm; c-di-GMP; histidine kinase; nitric oxide; two component signaling systems.
© 2023 The Author(s). Published by Portland Press Limited on behalf of the Biochemical Society.
Figures

Similar articles
-
NosP Signaling Modulates the NO/H-NOX-Mediated Multicomponent c-Di-GMP Network and Biofilm Formation in Shewanella oneidensis.Biochemistry. 2019 Dec 3;58(48):4827-4841. doi: 10.1021/acs.biochem.9b00706. Epub 2019 Nov 18. Biochemistry. 2019. PMID: 31682418 Free PMC article.
-
Discovery of Two Bacterial Nitric Oxide-Responsive Proteins and Their Roles in Bacterial Biofilm Regulation.Acc Chem Res. 2017 Jul 18;50(7):1633-1639. doi: 10.1021/acs.accounts.7b00095. Epub 2017 Jun 12. Acc Chem Res. 2017. PMID: 28605194 Free PMC article.
-
NosP Modulates Cyclic-di-GMP Signaling in Legionella pneumophila.Biochemistry. 2019 Oct 22;58(42):4325-4334. doi: 10.1021/acs.biochem.9b00618. Epub 2019 Oct 9. Biochemistry. 2019. PMID: 31576744 Free PMC article.
-
Towards Understanding the Molecular Basis of Nitric Oxide-Regulated Group Behaviors in Pathogenic Bacteria.J Innate Immun. 2019;11(3):205-215. doi: 10.1159/000494740. Epub 2018 Dec 17. J Innate Immun. 2019. PMID: 30557874 Free PMC article. Review.
-
[Networks involving quorum sensing, cyclic-di-GMP and nitric oxide on biofilm production in bacteria].Rev Argent Microbiol. 2014 Jul-Sep;46(3):242-55. doi: 10.1016/S0325-7541(14)70079-3. Epub 2014 Oct 15. Rev Argent Microbiol. 2014. PMID: 25444134 Review. Spanish.
Cited by
-
Two-Dimensional "Nanotanks" Release "Gas Bombs" through Photodynamic Cascades to Promote Diabetic Wound Healing.Biomater Res. 2024 Oct 29;28:0100. doi: 10.34133/bmr.0100. eCollection 2024. Biomater Res. 2024. PMID: 39474320 Free PMC article.
-
The histidine kinase NahK regulates denitrification and nitric oxide accumulation through RsmA in Pseudomonas aeruginosa.J Bacteriol. 2025 Jan 31;207(1):e0040824. doi: 10.1128/jb.00408-24. Epub 2024 Dec 11. J Bacteriol. 2025. PMID: 39660891 Free PMC article.
-
Harnessing Cyclic di-GMP Signaling: A Strategic Approach to Combat Bacterial Biofilm-Associated Chronic Infections.Curr Microbiol. 2025 Feb 5;82(3):118. doi: 10.1007/s00284-025-04091-7. Curr Microbiol. 2025. PMID: 39909925 Review.
-
Harnessing the Power of Our Immune System: The Antimicrobial and Antibiofilm Properties of Nitric Oxide.Microorganisms. 2024 Dec 10;12(12):2543. doi: 10.3390/microorganisms12122543. Microorganisms. 2024. PMID: 39770746 Free PMC article. Review.
-
Carbon source, cell density, and the microbial community control inhibition of V. cholerae surface colonization by environmental nitrate.mBio. 2025 Apr 9;16(4):e0406624. doi: 10.1128/mbio.04066-24. Epub 2025 Feb 25. mBio. 2025. PMID: 39998205 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

