Cobalt-Catalyzed Aerobic Aminocyclization of Unsaturated Amides for the Synthesis of Functionalized γ- and δ-Lactams
- PMID: 37610083
- PMCID: PMC10476186
- DOI: 10.1021/acs.orglett.3c02390
Cobalt-Catalyzed Aerobic Aminocyclization of Unsaturated Amides for the Synthesis of Functionalized γ- and δ-Lactams
Abstract
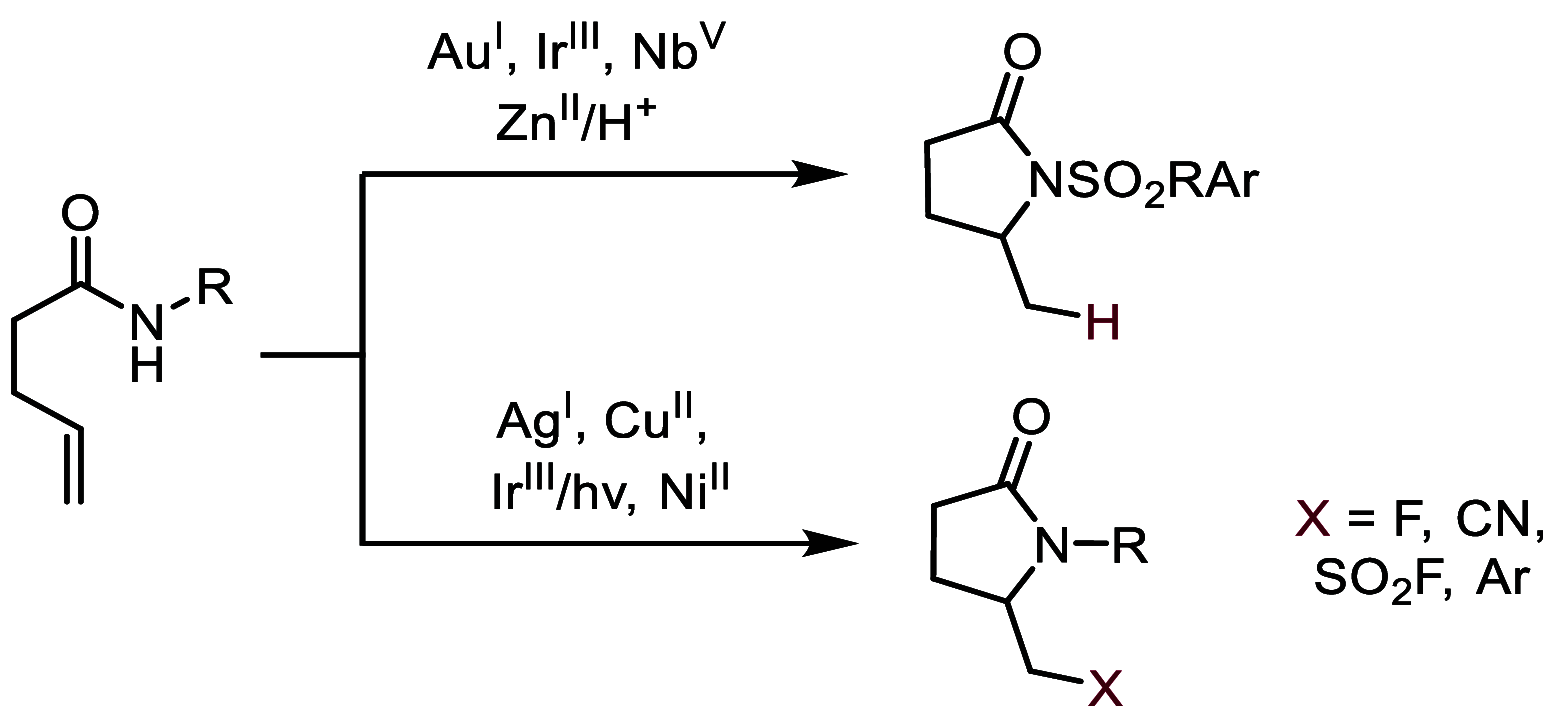
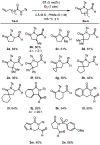
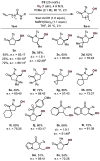
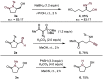
We report the cobalt-catalyzed aminocyclization of unsaturated N-acyl sulfonamides in the presence of oxygen to provide γ- and δ-lactam aldehydes. Use of an optically active cobalt catalyst resulted in the formation of enantiomerically enriched γ-and δ-lactam alcohols. The γ-lactam aldehydes and alcohols obtained were elaborated into useful building blocks.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Cobalt-Catalyzed Cyclization of Unsaturated N-Acyl Sulfonamides: a Diverted Mukaiyama Hydration Reaction.JACS Au. 2022 May 6;2(5):1071-1077. doi: 10.1021/jacsau.2c00186. eCollection 2022 May 23. JACS Au. 2022. PMID: 35647594 Free PMC article.
-
Mn- and Co-Catalyzed Aminocyclizations of Unsaturated Hydrazones Providing a Broad Range of Functionalized Pyrazolines.JACS Au. 2021 Jul 26;1(7):919-924. doi: 10.1021/jacsau.1c00176. Epub 2021 Jun 11. JACS Au. 2021. PMID: 34337605 Free PMC article.
-
Cobalt-Catalyzed Aerobic Oxidative Cleavage of Alkyl Aldehydes: Synthesis of Ketones, Esters, Amides, and α-Ketoamides.Chemistry. 2021 Jul 7;27(38):9737-9741. doi: 10.1002/chem.202101035. Epub 2021 May 27. Chemistry. 2021. PMID: 34010489
-
Organophosphorus reagents in organocatalysis: synthesis of optically active α-methylene-δ-lactones and δ-lactams.Chemistry. 2012 Aug 13;18(33):10348-54. doi: 10.1002/chem.201201325. Epub 2012 Jun 15. Chemistry. 2012. PMID: 22706879
-
Glycal-derived δ-hydroxy α,β-unsaturated aldehydes (Perlin aldehydes): versatile building blocks in organic synthesis.Chem Rev. 2013 May 8;113(5):3605-31. doi: 10.1021/cr200016m. Epub 2013 Feb 18. Chem Rev. 2013. PMID: 23419115 Review. No abstract available.
References
-
- Di Maso M. J.; Nepomuceno G. M.; St. Peter M. A.; Gitre H. H.; Martin K. S.; Shaw J. T. Synthesis of (±)-Bisavenanthramide B-6 by an Anionic Anhydride Mannich Reaction. Org. Lett. 2016, 18, 1740–1743. 10.1021/acs.orglett.6b00413. - DOI - PubMed
- Shen D.-Y.; Nguyen T. N.; Wu S.-J.; Shiao Y.-J.; Hung H.-Y.; Kuo P.-C.; Kuo D.-H.; Thang T. D.; Wu T.-S. γ- and δ-Lactams from the Leaves of Clausena lansium. J. Nat. Prod. 2015, 78, 2521–2530. 10.1021/acs.jnatprod.5b00148. - DOI - PubMed
- Fu T.-h.; McElroy W. T.; Shamszad M.; Martin S. F. Formal Syntheses of Naturally Occurring Welwitindolinones. Org. Lett. 2012, 14, 3834–3837. 10.1021/ol301424h. - DOI - PMC - PubMed
- Shenvi R. A.; Corey E. J. A Short and Efficient Synthesis of (−)-7-Methylomuralide, a Potent Proteasome Inhibitor. J. Am. Chem. Soc. 2009, 131, 5746–5747. 10.1021/ja901400q. - DOI - PMC - PubMed
- Okazaki Y.; Ishizuka A.; Ishihara A.; Nishioka T.; Iwamura H. New Dimeric Compounds of Avenanthramide Phytoalexin in Oats. J. Org. Chem. 2007, 72, 3830–3839. 10.1021/jo0701740. - DOI - PubMed
- Masse C. E.; Morgan A. J.; Adams J.; Panek J. S. Syntheses and Biological Evaluation of (+)-Lactacystin and Analogs. Eur. J. Org. Chem. 2000, 2000, 2513–2528. 10.1002/1099-0690(200007)2000:14<2513::AID-EJOC2513>3.0.CO;2-D. - DOI
- Caruano J.; Muccioli G. G.; Robiette R. Biologically active γ-lactams: synthesis and natural sources. Org. Biomol. Chem. 2016, 14, 10134–10156. 10.1039/C6OB01349J. - DOI - PubMed
-
- Fong A.; Ross M.; Boudreau J.; Nokhbeh R.; Tilbe K.; Lee H. Raja 42, a novel gamma lactam compound, is effective against Clostridioides difficile. PLoS One 2021, 16 (9), e025714310.1371/journal.pone.0257143. - DOI - PMC - PubMed
- Davies G. M; Hitchcock P. B; Loakes D.; Young D. W Synthesis of Reactive γ-Lactams Related to Penicillins and Cephalosporins. Tetrahedron Lett. 1996, 37, 5601–5604. 10.1016/0040-4039(96)01135-5. - DOI
-
- Velthuisen E. J.; Johns B. A.; Temelkoff D. P.; Brown K. W.; Danehower S. C. The design of 8-hydroxyquinoline tetracyclic lactams as HIV-1 integrase strand transfer inhibitors. Eur. J. Med. Chem. 2016, 117, 99–112. 10.1016/j.ejmech.2016.03.038. - DOI - PubMed
- Metífiot M.; Maddali K.; Johnson B. C.; Hare S.; Smith S. J.; Zhao X. Z.; Marchand C.; Burke T. R. Jr.; Hughes S. H.; Cherepanov P.; Pommier Y. Activities, Crystal Structures, and Molecular Dynamics of Dihydro-1H-isoindole Derivatives, Inhibitors of HIV-1 Integrase. ACS Chem. Biol. 2013, 8, 209–217. 10.1021/cb300471n. - DOI - PMC - PubMed
-
- O̅mura S.; Crump A. Lactacystin: first-in-class proteasome inhibitor still excelling and an exemplar for future antibiotic research. J. Antibiot. 2019, 72, 189–201. 10.1038/s41429-019-0141-8. - DOI - PMC - PubMed
- Fenical W.; Jensen P. R.; Palladino M. A.; Lam K. S.; Lloyd G. K.; Potts B. C. Discovery and development of the anticancer agent salinosporamide A (NPI-0052). Bioorg. Med. Chem. 2009, 17, 2175–2180. 10.1016/j.bmc.2008.10.075. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

