Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
- PMID: 37610090
- PMCID: PMC10482429
- DOI: 10.7554/eLife.90006
Coordination between ECM and cell-cell adhesion regulates the development of islet aggregation, architecture, and functional maturation
Abstract
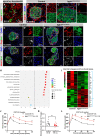
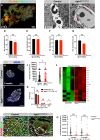
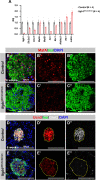
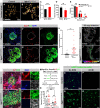
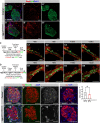
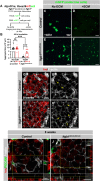
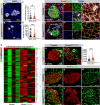
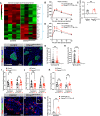
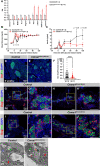
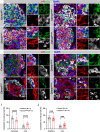
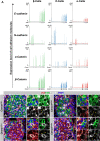
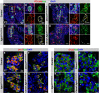
Pancreatic islets are three-dimensional cell aggregates consisting of unique cellular composition, cell-to-cell contacts, and interactions with blood vessels. Cell aggregation is essential for islet endocrine function; however, it remains unclear how developing islets establish aggregation. By combining genetic animal models, imaging tools, and gene expression profiling, we demonstrate that islet aggregation is regulated by extracellular matrix signaling and cell-cell adhesion. Islet endocrine cell-specific inactivation of extracellular matrix receptor integrin β1 disrupted blood vessel interactions but promoted cell-cell adhesion and the formation of larger islets. In contrast, ablation of cell-cell adhesion molecule α-catenin promoted blood vessel interactions yet compromised islet clustering. Simultaneous removal of integrin β1 and α-catenin disrupts islet aggregation and the endocrine cell maturation process, demonstrating that establishment of islet aggregates is essential for functional maturation. Our study provides new insights into understanding the fundamental self-organizing mechanism for islet aggregation, architecture, and functional maturation.
Keywords: ECM; cell adhesion; developmental biology; endocrine cells; integrin; islet; mouse; α-Catenin.
© 2023, Tixi, Maldonado, Chang et al.
Conflict of interest statement
WT, MM, YC, AC, WY, NP, MN, RH, SW, WH, PF, JK, MH, SD, HS No competing interests declared
Figures

Update of
References
-
- Adams MT, Dwulet JM, Briggs JK, Reissaus CA, Jin E, Szulczewski JM, Lyman MR, Sdao SM, Kravets V, Nimkulrat SD, Ponik SM, Merrins MJ, Mirmira RG, Linnemann AK, Benninger RK, Blum B. Reduced synchroneity of intra-islet Ca2+ oscillations in vivo in Robo-deficient β cells. eLife. 2021;10:e61308. doi: 10.7554/eLife.61308. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions

