The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs
- PMID: 37610204
- PMCID: PMC10653855
- DOI: 10.1128/mbio.01273-23
The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs
Retraction in
-
Retraction for Li et al., "The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs".mBio. 2024 Apr 10;15(4):e0010624. doi: 10.1128/mbio.00106-24. Epub 2024 Mar 22. mBio. 2024. PMID: 38517163 Free PMC article. No abstract available.
Corrected and republished in
-
Retracted and republished from: "The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs".mBio. 2024 May 8;15(5):e0017524. doi: 10.1128/mbio.00175-24. Epub 2024 Mar 29. mBio. 2024. PMID: 38551343 Free PMC article.
Abstract
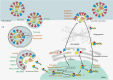
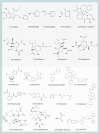
Influenza viruses (IVs) threaten global human health due to the high morbidity, infection, and mortality rates. Currently, the influenza drugs recommended by the FDA are oseltamivir, zanamivir, peramivir, and baloxavir marboxil. Notably, owing to the high variability of IVs, no drug exists that can effectively treat all types and subtypes of IVs. Moreover, the current trend of drug resistance is likely to continue as the viral genome is constantly mutating. Therefore, there is an urgent need to develop drugs related to the treatment of influenza to deal with the next pandemic. Here, we summarized the cutting-edge research in mechanism of action, inhibitory activity, and clinical efficacy of drugs that have been approved and drugs that are still in clinical trials for influenza treatment. We hope this review will provide up-to-date and comprehensive information on influenza antivirals and generate hypotheses for screens and development of new broad-spectrum influenza drugs in the near future.
Keywords: adverse event; antiviral drugs; clinical drugs; influenza virus; mechanism of action.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Retracted and republished from: "The current state of research on influenza antiviral drug development: drugs in clinical trial and licensed drugs".mBio. 2024 May 8;15(5):e0017524. doi: 10.1128/mbio.00175-24. Epub 2024 Mar 29. mBio. 2024. PMID: 38551343 Free PMC article.
-
Antiviral Activity of Probenecid and Oseltamivir on Influenza Virus Replication.Viruses. 2023 Nov 30;15(12):2366. doi: 10.3390/v15122366. Viruses. 2023. PMID: 38140606 Free PMC article.
-
Successful treatment with baloxavir marboxil of a patient with peramivir-resistant influenza A/H3N2 with a dual E119D/R292K substitution after allogeneic hematopoietic cell transplantation: a case report.BMC Infect Dis. 2020 Jul 6;20(1):478. doi: 10.1186/s12879-020-05205-1. BMC Infect Dis. 2020. PMID: 32631240 Free PMC article.
-
How to manage antivirals in critically ill patients with influenza?Clin Microbiol Infect. 2025 Jul;31(7):1157-1165. doi: 10.1016/j.cmi.2025.04.002. Epub 2025 Apr 7. Clin Microbiol Infect. 2025. PMID: 40204233 Review.
-
[Research progress of neuraminidase inhibitors for anti-influenza].Yao Xue Xue Bao. 2009 Sep;44(9):935-42. Yao Xue Xue Bao. 2009. PMID: 20055166 Review. Chinese.
Cited by
-
Impact of menopausal hormone therapy on influenza complications in women: a systematic assessment study.Ann Med. 2025 Dec;57(1):2534095. doi: 10.1080/07853890.2025.2534095. Epub 2025 Jul 17. Ann Med. 2025. PMID: 40676907 Free PMC article.
-
Deciphering the anti‑influenza potential of Eucommiae Cortex based on bioinformatics analysis: In silico and in vitro experiments.Exp Ther Med. 2025 Mar 27;29(5):106. doi: 10.3892/etm.2025.12856. eCollection 2025 May. Exp Ther Med. 2025. PMID: 40230620 Free PMC article.
-
Antiviral responses versus virus-induced cellular shutoff: a game of thrones between influenza A virus NS1 and SARS-CoV-2 Nsp1.Front Cell Infect Microbiol. 2024 Feb 5;14:1357866. doi: 10.3389/fcimb.2024.1357866. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38375361 Free PMC article. Review.
-
Advances and Challenges in Antiviral Development for Respiratory Viruses.Pathogens. 2024 Dec 31;14(1):20. doi: 10.3390/pathogens14010020. Pathogens. 2024. PMID: 39860981 Free PMC article. Review.
-
Sulfatide Binds to Influenza B Virus and Enhances Viral Replication.Viruses. 2025 Apr 5;17(4):530. doi: 10.3390/v17040530. Viruses. 2025. PMID: 40284974 Free PMC article.
References
-
- WHO . 2023. Influenza (seasonal). Available from: https://www.who.int/health-topics/influenza-seasonal
-
- CDC . 2023. Specific avian flu viruses. Available from: https://www.cdc.gov/flu/avianflu/specific-flu-viruses.htm
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

