Very early life microbiome and metabolome correlates with primary vaccination variability in children
- PMID: 37610205
- PMCID: PMC10654091
- DOI: 10.1128/msystems.00661-23
Very early life microbiome and metabolome correlates with primary vaccination variability in children
Abstract
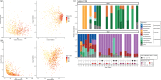
We show that simultaneous study of stool and nasopharyngeal microbiome reveals divergent timing and patterns of maturation, suggesting that local mucosal factors may influence microbiome composition in the gut and respiratory system. Antibiotic exposure in early life as occurs commonly, may have an adverse effect on vaccine responsiveness. Abundance of gut and/or nasopharyngeal bacteria with the machinery to produce lipopolysaccharide-a toll-like receptor 4 agonist-may positively affect future vaccine protection, potentially by acting as a natural adjuvant. The increased levels of serum phenylpyruvic acid in infants with lower vaccine-induced antibody levels suggest an increased abundance of hydrogen peroxide, leading to more oxidative stress in low vaccine-responding infants.
Keywords: antibiotics; children; metabolome; microbiome; vaccine response.
Conflict of interest statement
All authors who are/were employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, may hold stocks and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. Eduardo Gonzalez, Steven Schulz, and Michael Pichichero are employees of Rochester General Hospital, and that institution received a collaborative research grant from Merck & Co., Inc. for conducting the prospective trial, aspects of the laboratory work, data management, and analysis.
Figures

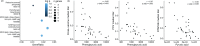
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

