Mirikizumab Pharmacokinetics in Patients with Moderately to Severely Active Ulcerative Colitis: Results from Phase III LUCENT Studies
- PMID: 37610533
- PMCID: PMC10520102
- DOI: 10.1007/s40262-023-01281-z
Mirikizumab Pharmacokinetics in Patients with Moderately to Severely Active Ulcerative Colitis: Results from Phase III LUCENT Studies
Abstract
Background and objective: Mirikizumab is a humanized anti-interleukin-23-p19 monoclonal antibody being developed for ulcerative colitis and Crohn's disease. This analysis characterized mirikizumab pharmacokinetics using phase II and III trial data from patients with moderately to severely active ulcerative colitis.
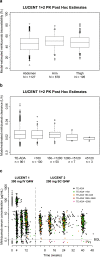
Methods: Serum pharmacokinetic data in patients receiving mirikizumab 50-1000 mg intravenously every 4 weeks as induction treatment and mirikizumab 200 mg subcutaneously every 4 or 12 weeks as maintenance treatment across three trials (N = 1362) were analyzed using non-linear mixed-effects modeling. Covariate effects on mirikizumab exposure were evaluated using simulation-based estimations.
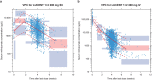
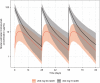
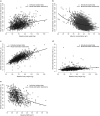
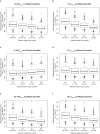
Results: Mirikizumab pharmacokinetics was best described by a linear two-compartment model with first-order absorption. Clearance, volume of distribution for central and peripheral compartments, and half-life were estimated at approximately 0.022 L/h (linear), 3.11 L and 1.69 L, and 9.5 days, respectively. Statistically significant effects of body weight and serum albumin levels on clearance, body weight on central and peripheral volumes of distribution, and body mass index on bioavailability were observed but effects were small relative to random inter-individual variability (% coefficient of variation: 18-64%). The subcutaneous bioavailability of mirikizumab was 48%.
Conclusions: Mirikizumab displayed pharmacokinetic characteristics typical of a monoclonal antibody where clearance increased with body weight and decreased with the albumin level, and bioavailability decreased with body mass index. These effects were small relative to random variability, indicating that a dose adjustment for patient factors is not required.
Clinical trial registration: ClinicalTrials.gov: NCT02589665 (28 October, 2015), NCT03518086 (8 May, 2018), NCT03524092 (14 May, 2018).
© 2023. The Author(s).
Conflict of interest statement
Laiyi Chua and Xin Cindy Zhang are employees and minor shareholders of Eli Lilly and Company. Stuart Friedrich is a former employee of Eli Lilly and Company.
Figures
References
-
- Lasa JS, Olivera PA, Danese S, Peyrin-Biroulet L. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170. doi: 10.1016/S2468-1253(21)00377-0. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

