Health Claims and Doses of Fish Oil Supplements in the US
- PMID: 37610733
- PMCID: PMC10448371
- DOI: 10.1001/jamacardio.2023.2424
Health Claims and Doses of Fish Oil Supplements in the US
Abstract
Importance: One in 5 US adults older than 60 years takes fish oil supplements often for heart health despite multiple randomized clinical trials showing no data for cardiovascular benefit for supplement-range doses. Statements on the supplement labels may influence consumer beliefs about health benefits.
Objectives: To evaluate health claims made on the labels of fish oil supplements in the US, and to examine doses of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) in commonly available formulations.
Design, setting, and participants: This cross-sectional study used data from labels of on-market fish oil (and nonfish ω-3 fatty acid) supplements obtained from the National Institutes of Health Dietary Supplement Label Database. The study was conducted and data analyzed from February to June 2022.
Main outcome and measures: The frequency and types of health claims made on fish oil labels (US Food and Drug Administration [FDA]-reviewed qualified health claim vs a structure/function claim) and the organ system referenced were evaluated. The total daily doses of combined EPA and DHA (EPA+DHA) were assessed for supplements from 16 leading manufacturers and retailers.
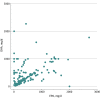
Results: Across 2819 unique fish oil supplements, 2082 (73.9%) made at least 1 health claim. Of these, only 399 (19.2%) used an FDA-approved qualified health claim; the rest (1683 [80.8%]) made only structure/function claims (eg, "promotes heart health"). Cardiovascular health claims were the most common (1747 [62.0%]). Across 16 leading brands/manufacturers, 255 fish oil supplements were identified. Among these, substantial variability was found in the daily dose of EPA (median [IQR], 340 [135-647] mg/d), DHA (median [IQR], 270 [140-500] mg/d), and total EPA+DHA (median [IQR], 600 [300-1100] mg/d). Only 24 of 255 supplements (9.4%) evaluated contained a daily dose of 2 g or more EPA+DHA.
Conclusions: Results of this cross-sectional study suggest that the majority of fish oil supplement labels make health claims, usually in the form of structure/function claims, that imply a health benefit across a variety of organ systems despite a lack of trial data showing efficacy. Significant heterogeneity exists in the daily dose of EPA+DHA in available supplements, leading to potential variability in safety and efficacy between supplements. Increasing regulation of dietary supplement labeling may be needed to prevent consumer misinformation.
Conflict of interest statement
Figures

References
-
- Mishra S, Stierman B, Gahche JJ, Potischman N. Dietary supplement use among adults: US, 2017–2018. Accessed May 17, 2022. https://www.cdc.gov/nchs/products/databriefs/db399.htm - PubMed
-
- Shalala DE, Henney JE. Regulations on statements made for dietary supplements concerning the effect of the product on the structure or function of the body. Accessed June 12, 2022. https://www.govinfo.gov/content/pkg/FR-2000-01-06/pdf/00-53.pdf
-
- Aung T, Halsey J, Kromhout D, et al. ; Omega-3 Treatment Trialists’ Collaboration . Associations of ω-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3(3):225-234. doi:10.1001/jamacardio.2017.5205 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

