Web-Based Video Education to Improve Uptake of Influenza Vaccination and Other Preventive Health Recommendations in Adults With Inflammatory Bowel Disease: Randomized Controlled Trial of Project PREVENT
- PMID: 37610821
- PMCID: PMC10483303
- DOI: 10.2196/42921
Web-Based Video Education to Improve Uptake of Influenza Vaccination and Other Preventive Health Recommendations in Adults With Inflammatory Bowel Disease: Randomized Controlled Trial of Project PREVENT
Abstract
Background: Patients with inflammatory bowel disease (IBD) are at increased risk of infections, bone fractures, and skin cancers.
Objective: We developed preventive health videos using a patient-centered approach and tested their impact on preventive health uptake.
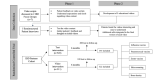
Methods: Five animated videos explaining preventive health recommendations in IBD were iteratively developed with patient-centered focus groups and interviews. A randomized controlled trial was then conducted in a web-based IBD cohort to test the impact of video- versus text-based educational interventions. The primary outcome was receipt of the influenza vaccine. Secondary outcomes included intention to receive other preventive health services.
Results: Five animated videos were developed with patient input. A total of 1056 patients with IBD were then randomized to receive the video (n=511) or text-only (n=545) interventions; 55% (281/511) of the video group and 57% (311/545) of the text-only group had received their influenza vaccine in the prior year. Immediately after the intervention, 73% (502/683) of patients reported their intention to receive the vaccine, with no difference by the type of intervention (75%, 231/307, for the video group and 72%, 271/376, for the text-only group). The proportion of patients who actually received the influenza vaccine after the intervention also did not differ by messaging type (P=.07). The strongest predictor of both intention to receive and actual receipt of the influenza vaccine was prior influenza vaccination. Older age was also associated with a higher likelihood of the intention to receive (age 36-75 years relative to 18-35 years; P=.006) and actual receipt (age >75 years relative to 18-35 years; P=.05) of the influenza vaccine.
Conclusions: The proportion of patients receiving the influenza vaccine was high in both groups, but there was no difference in receipt of or in the intention to receive preventive health recommendations by type of messaging. Notably, a portion of patients in both groups had intended to be vaccinated but did not ultimately receive the vaccine. Further evaluation of patient-education strategies is warranted to improve preventive health uptake among patients with IBD.
Trial registration: ClinicalTrials.gov NCT05997537; https://clinicaltrials.gov/ct2/show/NCT05997537.
Keywords: adults; bone; cancer; development; disease; education; infections; inflammation; inflammatory bowel disease (IBD); influenza; intervention; interview; patient; preventative; prevention; risk; vaccination.
©Millie D Long, Welmoed K van Deen, Laura Weisbein, Carine Khalil, Keren L Appel, Xian Zhang, Wenli Chen, Lori Zubrod, Robbie Maris, Afsoon Ghafari, Taylor Dupuy, Christina Y Ha, Brennan M R Spiegel, Christopher V Almario, Gil Y Melmed. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 23.08.2023.
Conflict of interest statement
Conflicts of Interest: WKvD has consulted for the Crohn and Colitis Foundation. GYM has consulted for AbbVie, Arena Pharmaceuticals, Boehringer-Ingelheim, Bristol-Meyers Squibb/Celgene, Entasis, Janssen, Medtronic, Pfizer, Prometheus Labs, Samsung Bioepis, Shionogi, Takeda, and Techlab, and has received research funding from Pfizer for an unrelated investigator-initiated study. BMRS reports grants from Takeda, Ironwood, and Amgen, and advisory board participation for Ardelyx, Exact Sciences, and Ferring. CYH is on the advisory board for Abbvie, Janssen, Pfizer, Takeda, Roivant, BMS, and Lilly, and receives grant support from Pfiser and Helmsley Charitable Trust. MDL has consuled for AbbVie, Takeda, Janssen, Pfizer, Lilly, BMS, Prometheus, Roche/Genentech, and Target RWE. GYM is a consultant for Abbvie, Arena, Briatol Myers Squibb, Celgene, Janssen, Medtronic, Pfizer, Takeda, Viatris, Fresenius Kabi, Prometheus Labs, Oshi, and Dieta. Research funding for this project was provided by Pfizer, but Pfizer had no role in study design, execution, interpretation of results, or decision to publish. No other conflicts are reported.
Figures

References
-
- Vernier-Massouille G, Balde M, Salleron J, Turck D, Dupas JL, Mouterde O, Merle V, Salomez JL, Branche J, Marti R, Lerebours ?, Cortot A, Gower-Rousseau C, Colombel JF. Natural history of pediatric Crohn's disease: a population-based cohort study. Gastroenterology. 2008 Oct;135(4):1106–13. doi: 10.1053/j.gastro.2008.06.079.S0016-5085(08)01199-2 - DOI - PubMed
-
- Van Limbergen J, Russell RK, Drummond HE, Aldhous MC, Round NK, Nimmo ER, Smith L, Gillett PM, McGrogan P, Weaver LT, Bisset WM, Mahdi G, Arnott ID, Satsangi J, Wilson DC. Definition of phenotypic characteristics of childhood-onset inflammatory bowel disease. Gastroenterology. 2008 Oct;135(4):1114–22. doi: 10.1053/j.gastro.2008.06.081. https://core.ac.uk/reader/28967390?utm_source=linkout S0016-5085(08)01201-8 - DOI - PubMed
-
- Ferguson A, Sedgwick DM, Drummond J. Morbidity of juvenile onset inflammatory bowel disease: effects on education and employment in early adult life. Gut. 1994 May;35(5):665–8. doi: 10.1136/gut.35.5.665. https://gut.bmj.com/lookup/pmidlookup?view=long&pmid=8200562 - DOI - PMC - PubMed
-
- Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther. 2002 Sep;16(9):1603–9. doi: 10.1046/j.1365-2036.2002.01323.x. https://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pu... 1323 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

