Severe aortic stenosis detection by deep learning applied to echocardiography
- PMID: 37611002
- PMCID: PMC11004929
- DOI: 10.1093/eurheartj/ehad456
Severe aortic stenosis detection by deep learning applied to echocardiography
Abstract
Background and aims: Early diagnosis of aortic stenosis (AS) is critical to prevent morbidity and mortality but requires skilled examination with Doppler imaging. This study reports the development and validation of a novel deep learning model that relies on two-dimensional (2D) parasternal long axis videos from transthoracic echocardiography without Doppler imaging to identify severe AS, suitable for point-of-care ultrasonography.
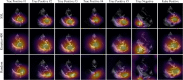
Methods and results: In a training set of 5257 studies (17 570 videos) from 2016 to 2020 [Yale-New Haven Hospital (YNHH), Connecticut], an ensemble of three-dimensional convolutional neural networks was developed to detect severe AS, leveraging self-supervised contrastive pretraining for label-efficient model development. This deep learning model was validated in a temporally distinct set of 2040 consecutive studies from 2021 from YNHH as well as two geographically distinct cohorts of 4226 and 3072 studies, from California and other hospitals in New England, respectively. The deep learning model achieved an area under the receiver operating characteristic curve (AUROC) of 0.978 (95% CI: 0.966, 0.988) for detecting severe AS in the temporally distinct test set, maintaining its diagnostic performance in geographically distinct cohorts [0.952 AUROC (95% CI: 0.941, 0.963) in California and 0.942 AUROC (95% CI: 0.909, 0.966) in New England]. The model was interpretable with saliency maps identifying the aortic valve, mitral annulus, and left atrium as the predictive regions. Among non-severe AS cases, predicted probabilities were associated with worse quantitative metrics of AS suggesting an association with various stages of AS severity.
Conclusion: This study developed and externally validated an automated approach for severe AS detection using single-view 2D echocardiography, with potential utility for point-of-care screening.
Keywords: Aortic stenosis; Deep learning; Digital health; Echocardiography.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures