Nitrogen-15 dynamic nuclear polarization of nicotinamide derivatives in biocompatible solutions
- PMID: 37611105
- PMCID: PMC10446501
- DOI: 10.1126/sciadv.add3643
Nitrogen-15 dynamic nuclear polarization of nicotinamide derivatives in biocompatible solutions
Abstract
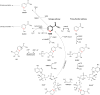
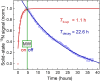
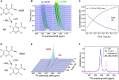
Dissolution dynamic nuclear polarization (dDNP) increases the sensitivity of magnetic resonance imaging by more than 10,000 times, enabling in vivo metabolic imaging to be performed noninvasively in real time. Here, we are developing a group of dDNP polarized tracers based on nicotinamide (NAM). We synthesized 1-15N-NAM and 1-15N nicotinic acid and hyperpolarized them with dDNP, reaching (13.0 ± 1.9)% 15N polarization. We found that the lifetime of hyperpolarized 1-15N-NAM is strongly field- and pH-dependent, with T1 being as long as 41 s at a pH of 12 and 1 T while as short as a few seconds at neutral pH and fields below 1 T. The remarkably short 1-15N lifetime at low magnetic fields and neutral pH drove us to establish a unique pH neutralization procedure. Using 15N dDNP and an inexpensive rodent imaging probe designed in-house, we acquired a 15N MRI of 1-15N-NAM (previously hyperpolarized for more than an hour) in less than 1 s.
Figures



Similar articles
-
Signal enhancement of hyperpolarized 15 N sites in solution-increase in solid-state polarization at 3.35 T and prolongation of relaxation in deuterated water mixtures.NMR Biomed. 2022 Nov;35(11):e4787. doi: 10.1002/nbm.4787. Epub 2022 Jul 14. NMR Biomed. 2022. PMID: 35704397 Free PMC article.
-
Nuclear Overhauser spectroscopy in hyperpolarized water - chemical vs. magnetic exchange.Chem Commun (Camb). 2022 Oct 18;58(83):11661-11664. doi: 10.1039/d2cc03735a. Chem Commun (Camb). 2022. PMID: 36169286 Free PMC article.
-
Influence of DNP Polarizing Agents on Biochemical Processes: TEMPOL in Transient Ischemic Stroke.ACS Chem Neurosci. 2023 Sep 6;14(17):3013-3018. doi: 10.1021/acschemneuro.3c00137. Epub 2023 Aug 21. ACS Chem Neurosci. 2023. PMID: 37603041 Free PMC article.
-
Hyperpolarized MRI - An Update and Future Perspectives.Semin Nucl Med. 2022 May;52(3):374-381. doi: 10.1053/j.semnuclmed.2021.09.001. Epub 2021 Nov 14. Semin Nucl Med. 2022. PMID: 34785033 Review.
-
Hyperpolarized 13C metabolic imaging using dissolution dynamic nuclear polarization.J Magn Reson Imaging. 2012 Dec;36(6):1314-28. doi: 10.1002/jmri.23753. J Magn Reson Imaging. 2012. PMID: 23165733 Review.
Cited by
-
Maximizing NMR Sensitivity: A Guide to Receiver Gain Adjustment.NMR Biomed. 2025 Jun;38(6):e70046. doi: 10.1002/nbm.70046. NMR Biomed. 2025. PMID: 40342208 Free PMC article.
-
Analysis of chemical exchange in iridium N-heterocyclic carbene complexes using heteronuclear parahydrogen-enhanced NMR.Commun Chem. 2024 Dec 3;7(1):286. doi: 10.1038/s42004-024-01376-z. Commun Chem. 2024. PMID: 39627452 Free PMC article.
-
Toward Next-Generation Molecular Imaging with a Clinical Low-Field (0.064 T) Point-of-Care MRI Scanner.Anal Chem. 2024 Jun 25;96(25):10348-10355. doi: 10.1021/acs.analchem.4c01299. Epub 2024 Jun 10. Anal Chem. 2024. PMID: 38857182 Free PMC article.
-
Yeast Solutions and Hyperpolarization Enable Real-Time Observation of Metabolized Substrates Even at Natural Abundance.Anal Chem. 2024 Oct 29;96(43):17135-17144. doi: 10.1021/acs.analchem.4c02419. Epub 2024 Oct 15. Anal Chem. 2024. PMID: 39405516 Free PMC article.
-
Cross-Polarization of Insensitive Nuclei from Water Protons for Detection of Protein-Ligand Binding.J Am Chem Soc. 2024 Sep 11;146(36):24754-24758. doi: 10.1021/jacs.4c08241. Epub 2024 Sep 3. J Am Chem Soc. 2024. PMID: 39225120 Free PMC article.
References
-
- Grundy S. M., Pre-diabetes, metabolic syndrome, and cardiovascular risk. J. Am. Coll. Cardiol. 59, 635–643 (2012). - PubMed
-
- Kishnani P. S., Howell R. R., Pompe disease in infants and children. J. Pediatr. 144, S35–S43 (2004). - PubMed
-
- Haase A., Frahm J., Matthaei D., Hänicke W., Merboldt K.-D., FLASH imaging: Rapid NMR imaging using low flip-angle pulses. J. Magn. Reson. 213, 533–541 (1986). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

