Superior sulcus non-small cell lung cancers (Pancoast tumors): Current outcomes after multidisciplinary management
- PMID: 37611845
- PMCID: PMC11229055
- DOI: 10.1016/j.jtcvs.2023.08.023
Superior sulcus non-small cell lung cancers (Pancoast tumors): Current outcomes after multidisciplinary management
Abstract
Objective: Despite neoadjuvant chemoradiotherapy, Pancoast tumors still present surgical and oncologic challenges. To optimize outcomes, we used a multidisciplinary care paradigm with medical and radiation oncology, and involvement of spine neurosurgery for most T3 and all T4 tumors. Spine neurosurgery permitted resection of transverse process for T3 and vertebral body resection for T4 tumors.
Methods: Retrospective analysis of single institution, prospective database of patients undergoing resection for cT3 4M0 Pancoast tumors. Patients were grouped as cT3 with combined resection with spine neurosurgery (T3 Neuro), cT3 without spine neurosurgery (T3 NoNeuro), and cT4. Overall survival, progression-free survival were analyzed by Kaplan-Meier and compared between groups using log-rank test. Cumulative incidence of local-regional and distant recurrence were compared using Gray test. P value <.05 was considered significant.
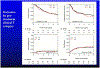
Results: From 2000 to 2021, 155 patients underwent surgery: median age was 58 years, and 81 were (52%) men. Most patients received neoadjuvant platinum-based neoadjuvant chemoradiotherapy (n = 127 [82%]). Operations were 48 cT3 Neuro, 41 cT3 NoNeuro, 66 cT4. R0 resection was achieved in 49 (94%) cT3 NoNeuro, 35 (85%) cT3 Neuro, and 57 (86%) cT4 patients (P = .4). Complete or major pathologic response occurred in 71 (55%) patients. Lower local-regional cumulative incidence was seen in cT3 Neuro versus cT3 NoNeuro (P = .05) and after major pathologic response. Overall survival and progression-free survival were associated with complete response, pathologic stage, and nodal status but not cT category.
Conclusions: This treatment paradigm was associated with a high frequency of R0 resection, complete response, and major pathologic response. cT3 and cT4 tumors had similar outcomes. Novel therapies are needed to improve complete response.
Keywords: Pancoast tumors; chemotherapy; radiation; surgery.
Copyright © 2023. Published by Elsevier Inc.
Conflict of interest statement
Conflict of Interest Statement Dr Rusch is a member of the Data Safety and Monitoring Committee for the Mesothelioma and Radical Surgery 2 (MARS2) and Radical Management of Advanced Non-Ssmall Cell Lung Cancer (RAMON) trials (in the United Kingdom) and serves as co-chair of the National Cancer Institute Thoracic Staging Malignancy Committee. She reports institutional funding from Genentech unrelated to this project. Dr Chen reports institutional funding from the National Institutes of Health (NIH), stock ownership of Nordik, Quest, and DOCS, and is a recipient of the American Society of Clinical Oncology young investigator award. Dr Chaft serves as an advisor to Genentech/Roche, AstraZeneca/MedImmune, Merck, Bristol Myers Squibb, Flame Biosciences, Janssen Oncology, Guardant Health, Regeneron/Sanofi, and Novartis. She reports research funding from Genentech/Roche, Bristol Myers Squibb, AstraZeneca/MedImmune, and Merck unrelated to this project. Dr Rimner is vice president of the International Thymic Malignancies Interest Group, and a board member of the International Mesothelioma Interest Group. He reports institutional funding from The Joanne & John DallePezze Foundation, Varian Medical Systems, AstraZeneca, Merck, Boehringer Ingelheim, Pfizer, NIH, and consulting fees from AstraZeneca, Merck and More Health. Dr Travis serves as an unpaid consultant for the Lung Cancer Mutation Consortium 3 (LCMC3) and Lung Cancer Mutation Consortium 4 (LCMC4) trials supported by Genentech. Dr Isbell reports stock ownership in LumaCyte and is a consultant/advisory board member for Roche Genentech. Dr Molena serves on a steering committee for AstraZeneca and as a consultant for Johnson & Johnson, Bristol Myers Squibb, Merck, and Genentech. Dr Park has served as a proctor for Intuitive Surgical and as a consultant for COTA. Dr Jones serves as a consultant for AstraZeneca and is on a clinical trial steering committee for Merck. All other authors reported no conflicts of interest. The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Figures


Comment in
-
Discussion to: Superior sulcus non-small cell lung cancers (Pancoast tumors): Current outcomes after multidisciplinary management.J Thorac Cardiovasc Surg. 2023 Dec;166(6):1488-1489. doi: 10.1016/j.jtcvs.2023.09.024. Epub 2023 Oct 11. J Thorac Cardiovasc Surg. 2023. PMID: 37831023 No abstract available.
References
-
- Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Johnson D, et al. Induction chemoradiation and surgical resection for non-small cell lung carcinomas of the superior sulcus: Initial results of Southwest Oncology Group trial 9416 (Intergroup trial 0160). J Thorac Cardiovasc Surg 2001;121:472–83. - PubMed
-
- Rusch VW, Giroux DJ, Kraut MJ, Crowley J, Hazuka M, Winton T, et al. Induction chemoradiation and surgical resection for superior sulcus non-small cell lung carcinomas: Long-term results of Southwest Oncology Group trial 9416 (Intergroup trial 0160). J Clin Oncol 2007;25:313–8. - PubMed
-
- Masaoka A, Ito Y, Yasumitsu T. Anterior approach for tumor of the superior sulcus. J Thorac Cardiovasc Surg 1979;78:413–5. - PubMed
-
- Bolton WD, Rice DC, Goodyear A, Correa AM, Erasmus J, Hofstetter W, et al. Superior sulcus tumors with vertebral body involvement: A multimodality approach. J Thorac Cardiovasc Surg 2009;137(6):1379–87. - PubMed
-
- Sundaresan N, Hilaris BS, Martini N. The combined neurosurgical-thoracic management of superior sulcus tumors. J Clin Oncol 1987;5:1739–45. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

