Studying Hair Growth Cycle and its Effects on Mouse Skin
- PMID: 37612030
- PMCID: PMC10584012
- DOI: 10.1016/j.jid.2023.04.015
Studying Hair Growth Cycle and its Effects on Mouse Skin
Abstract
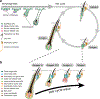
Researchers should be aware that hair growth cycle drives prominent molecular, cellular, and morphological changes to the entire skin. Thus, hair growth constitutes a major experimental variable that influences the interpretation of dermatological studies. Hair growth in mice is neither asynchronous nor fully synchronized; rather, it occurs in waves that dynamically propagate across the skin. In consequence, any given area of mouse skin can contain hair follicles in different stages of the cycle in close physical proximity. Furthermore, hair growth waves in mice are initiated by probabilistic events at different time points and across stochastic locations. The consequence of such stochasticity is that precise patterns of hair growth waves differ from mouse to mouse, even in littermates of the same sex. However, such physiological stochasticity is commonly misconstrued as a significant hair growth phenotype in mutant mice or in drug-treated mice. The purpose of this article is to provide a set of guidelines for designing reliably interpretable murine studies on hair growth and to highlight key experimental caveats to be avoided. It also informs on how to account for and minimize the impact of physiological hair cycle differences when designing and interpreting nonhair growth dermatological studies in mice.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
The authors declare that they have no competing interests.
Figures

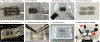
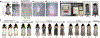

References
-
- Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J Invest Dermatol 2011;131(2):518–28. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

