Type-2 CD8+ T-cell formation relies on interleukin-33 and is linked to asthma exacerbations
- PMID: 37612281
- PMCID: PMC10447424
- DOI: 10.1038/s41467-023-40820-x
Type-2 CD8+ T-cell formation relies on interleukin-33 and is linked to asthma exacerbations
Abstract
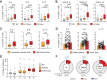
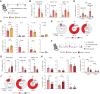
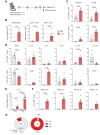
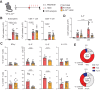
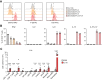
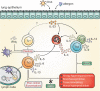
CD4+ T helper 2 (Th2) cells and group 2 innate lymphoid cells are considered the main producers of type-2 cytokines that fuel chronic airway inflammation in allergic asthma. However, CD8+ cytotoxic T (Tc) cells - critical for anti-viral defense - can also produce type-2 cytokines (referred to as 'Tc2' cells). The role of Tc cells in asthma and virus-induced disease exacerbations remains poorly understood, including which micro-environmental signals and cell types promote Tc2 cell formation. Here we show increased circulating Tc2 cell abundance in severe asthma patients, reaching peak levels during exacerbations and likely emerging from canonical IFNγ+ Tc cells through plasticity. Tc2 cell abundance is associated with increased disease burden, higher exacerbations rates and steroid insensitivity. Mouse models of asthma recapitulate the human disease by showing extensive type-2 skewing of lung Tc cells, which is controlled by conventional type-1 dendritic cells and IFNγ. Importantly, we demonstrate that the alarmin interleukin-33 (IL-33) critically promotes type-2 cytokine production by lung Tc cells in experimental allergic airway inflammation. Our data identify Tc cells as major producers of type-2 cytokines in severe asthma and during exacerbations that are remarkably sensitive to alterations in their inflammatory tissue micro-environment, with IL-33 emerging as an important regulator of Tc2 formation.
© 2023. Springer Nature Limited.
Conflict of interest statement
G.J.B. and G.A.T. declare the following competing interests: G.J.B. has received grant/research support for consultations and/or speaking at conferences from Novartis, GSK, AstraZeneca, ALK, Teva, Sanofi, and Chiesi. G.A.T. has received research support and consultation fees from OM Pharma, AstraZeneca, and Chiesi, all paid to a research foundation. The remaining authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

