An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis
- PMID: 37612411
- PMCID: PMC10474074
- DOI: 10.1038/s12276-023-01077-y
An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis
Abstract
Ferroptosis is a form of regulated cell death characterized by iron-dependent lipid peroxidation. This process contributes to cellular and tissue damage in various human diseases, such as cardiovascular diseases, neurodegeneration, liver disease, and cancer. Although polyunsaturated fatty acids (PUFAs) in membrane phospholipids are preferentially oxidized, saturated/monounsaturated fatty acids (SFAs/MUFAs) also influence lipid peroxidation and ferroptosis. In this review, we first explain how cells differentially synthesize SFA/MUFAs and PUFAs and how they control fatty acid pools via fatty acid uptake and β-oxidation, impacting ferroptosis. Furthermore, we discuss how fatty acids are stored in different lipids, such as diacyl or ether phospholipids with different head groups; triglycerides; and cholesterols. Moreover, we explain how these fatty acids are released from these molecules. In summary, we provide an integrated view of the diverse and dynamic metabolic processes in the context of ferroptosis by revisiting lipidomic studies. Thus, this review contributes to the development of therapeutic strategies for ferroptosis-related diseases.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
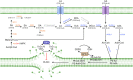
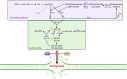
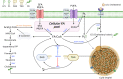
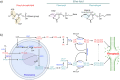
Similar articles
-
Lipid availability influences ferroptosis sensitivity in cancer cells by regulating polyunsaturated fatty acid trafficking.Cell Chem Biol. 2025 Mar 20;32(3):408-422.e6. doi: 10.1016/j.chembiol.2024.09.008. Epub 2024 Oct 22. Cell Chem Biol. 2025. PMID: 39442523
-
A tale of two lipids: Lipid unsaturation commands ferroptosis sensitivity.Proteomics. 2023 Mar;23(6):e2100308. doi: 10.1002/pmic.202100308. Epub 2023 Jan 13. Proteomics. 2023. PMID: 36398995 Review.
-
Polyunsaturated Fatty Acids Drive Lipid Peroxidation during Ferroptosis.Cells. 2023 Mar 4;12(5):804. doi: 10.3390/cells12050804. Cells. 2023. PMID: 36899940 Free PMC article. Review.
-
Ether lipid deficiency disrupts lipid homeostasis leading to ferroptosis sensitivity.PLoS Genet. 2022 Sep 30;18(9):e1010436. doi: 10.1371/journal.pgen.1010436. eCollection 2022 Sep. PLoS Genet. 2022. PMID: 36178986 Free PMC article.
-
Lipid availability influences ferroptosis sensitivity in cancer cells by regulating polyunsaturated fatty acid trafficking.bioRxiv [Preprint]. 2024 May 9:2024.05.06.592780. doi: 10.1101/2024.05.06.592780. bioRxiv. 2024. Update in: Cell Chem Biol. 2025 Mar 20;32(3):408-422.e6. doi: 10.1016/j.chembiol.2024.09.008. PMID: 38766165 Free PMC article. Updated. Preprint.
Cited by
-
Acyl-CoA thioesterase 8 induces gemcitabine resistance via regulation of lipid metabolism and antiferroptotic activity in pancreatic ductal adenocarcinoma.Acta Pharmacol Sin. 2025 Jun;46(6):1742-1756. doi: 10.1038/s41401-025-01477-y. Epub 2025 Feb 12. Acta Pharmacol Sin. 2025. PMID: 39939803 Free PMC article.
-
Promiscuous enzyme SQOR in cellular metabolism and ferroptosis regulation.BMB Rep. 2025 Jun;58(6):233-237. doi: 10.5483/BMBRep.2025-0019. BMB Rep. 2025. PMID: 40495478 Free PMC article. Review.
-
PNKP targeting engages the autophagic machinery through STING and STAT3 to potentiate ferroptosis and chemotherapy in TNBC.Redox Biol. 2025 Jul 22;86:103775. doi: 10.1016/j.redox.2025.103775. Online ahead of print. Redox Biol. 2025. PMID: 40743845 Free PMC article.
-
Lipid metabolism disorder in diabetic kidney disease.Front Endocrinol (Lausanne). 2024 Apr 29;15:1336402. doi: 10.3389/fendo.2024.1336402. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 38742197 Free PMC article. Review.
-
Study on the Mechanism of Formononetin Against Hepatocellular Carcinoma: Regulating Metabolic Pathways of Ferroptosis and Cell Cycle.Int J Mol Sci. 2025 Mar 13;26(6):2578. doi: 10.3390/ijms26062578. Int J Mol Sci. 2025. PMID: 40141219 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

