Reef-building corals farm and feed on their photosynthetic symbionts
- PMID: 37612503
- PMCID: PMC10468396
- DOI: 10.1038/s41586-023-06442-5
Reef-building corals farm and feed on their photosynthetic symbionts
Erratum in
-
Author Correction: Reef-building corals farm and feed on their photosynthetic symbionts.Nature. 2023 Sep;621(7980):E44. doi: 10.1038/s41586-023-06584-6. Nature. 2023. PMID: 37697059 Free PMC article. No abstract available.
Abstract
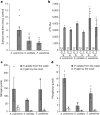
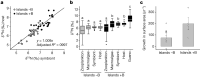
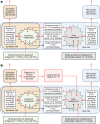
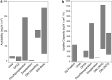
Coral reefs are highly diverse ecosystems that thrive in nutrient-poor waters, a phenomenon frequently referred to as the Darwin paradox1. The energy demand of coral animal hosts can often be fully met by the excess production of carbon-rich photosynthates by their algal symbionts2,3. However, the understanding of mechanisms that enable corals to acquire the vital nutrients nitrogen and phosphorus from their symbionts is incomplete4-9. Here we show, through a series of long-term experiments, that the uptake of dissolved inorganic nitrogen and phosphorus by the symbionts alone is sufficient to sustain rapid coral growth. Next, considering the nitrogen and phosphorus budgets of host and symbionts, we identify that these nutrients are gathered through symbiont 'farming' and are translocated to the host by digestion of excess symbiont cells. Finally, we use a large-scale natural experiment in which seabirds fertilize some reefs but not others, to show that the efficient utilization of dissolved inorganic nutrients by symbiotic corals established in our laboratory experiments has the potential to enhance coral growth in the wild at the ecosystem level. Feeding on symbionts enables coral animals to tap into an important nutrient pool and helps to explain the evolutionary and ecological success of symbiotic corals in nutrient-limited waters.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Corals have algal friends for dinner.Nature. 2023 Aug;620(7976):951-952. doi: 10.1038/d41586-023-02593-7. Nature. 2023. PMID: 37612489 No abstract available.
References
-
- Rougerie, F. in The French-Speaking Pacific: Population, Environment and Eevelopment Issues (ed. Jost, C.) 163–171 (Boombana, 1998).
-
- Falkowski, P. G., Dubinsky, Z., Muscatine, L. & Porter, J. W. Light and the bioenergetics of a symbiotic coral. Bioscience34, 705–709 (1984).
-
- Muscatine, L., Falkowski, P., Porter, J. & Dubinsky, Z. Fate of photosynthetic fixed carbon in light-and shade-adapted colonies of the symbiotic coral Stylophora pistillata. Proc. R. Soc. Lond. B222, 181–202 (1984).
-
- Godinot, C., Ferrier-Pagès, C. & Grover, R. Control of phosphate uptake by zooxanthellae and host cells in the scleractinian coral Stylophora pistillata. Limnol. Oceanogr.54, 1627–1633 (2009).
-
- Sutton, D. & Hoegh-Guldberg, O. Host–zooxanthella interactions in four temperate marine invertebrate symbioses: assessment of effect of host extracts on symbionts. Biol. Bull.178, 175–186 (1990). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

