mRNA vaccine in cancer therapy: Current advance and future outlook
- PMID: 37612832
- PMCID: PMC10447885
- DOI: 10.1002/ctm2.1384
mRNA vaccine in cancer therapy: Current advance and future outlook
Abstract
Messenger ribonucleic acid (mRNA) vaccines are a relatively new class of vaccines that have shown great promise in the immunotherapy of a wide variety of infectious diseases and cancer. In the past 2 years, SARS-CoV-2 mRNA vaccines have contributed tremendously against SARS-CoV2, which has prompted the arrival of the mRNA vaccine research boom, especially in the research of cancer vaccines. Compared with conventional cancer vaccines, mRNA vaccines have significant advantages, including efficient production of protective immune responses, relatively low side effects and lower cost of acquisition. In this review, we elaborated on the development of cancer vaccines and mRNA cancer vaccines, as well as the potential biological mechanisms of mRNA cancer vaccines and the latest progress in various tumour treatments, and discussed the challenges and future directions for the field.
Keywords: cancer; cancer vaccine; immunology; immunotherapy; mRNA vaccine.
© 2023 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


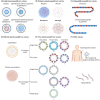
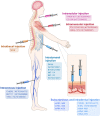
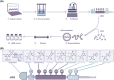


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
