Fecal occult blood and calprotectin testing to prioritize primary care patients for colonoscopy referral: The advantage study
- PMID: 37614054
- PMCID: PMC10493338
- DOI: 10.1002/ueg2.12446
Fecal occult blood and calprotectin testing to prioritize primary care patients for colonoscopy referral: The advantage study
Abstract
Background: Colonoscopy is the gold standard for colorectal cancer (CRC) diagnosis and screening, but endoscopy services are usually overburdened. This study aims to investigate the usefulness of fecal hemoglobin (fHb) and calprotectin (FC) for the identification of patients with high probability of CRC who need urgent referral.
Methods: In a multicenter prospective study, we enrolled symptomatic patients referred from primary care for colonoscopy. Prior to bowel preparation, fHb and FC quantitative tests were performed. The diagnostic performance was estimated for each biomarker/combination. We built a multivariable predictive model based on logistic regression, translated to a nomogram and a risk calculator to assist clinicians in the decision-making process.
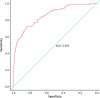
Results: The study included 1224 patients, of whom 69 (5.6%) had CRC. At the fHb cut-offs of >0 and 10 μg/g, the negative predictive values for CRC were 98.8% (95% confidence interval 97.8%-99.3%) and 98.6% (95%CI 97.7%-99.1%), and the sensitivities were 85.5% (95%CI 75.0%-92.8%) and 79.7% (95%CI 68.3%-88.4%), respectively. When we added the cut-off of 150 μg/g of FC to both fHb thresholds, the sensitivity of fecal tests improved. In the multivariate logistic regression model, the concentration of fHb was an independent predictor for CRC; age and gender were also independently associated with CRC.
Conclusions: fHb and FC are useful as part of a triage tool to identify those symptomatic patients with high probability of CRC. This can be easily applied by physicians to prioritize high-risk patients for urgent colonoscopy.
Keywords: colon cancer; colonoscopy; fecal calprotectin; fecal hemoglobin; fecal occult blood.
© 2023 The Authors. United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
FB received endoscopic equipment on loan of Fujifilm, received an honorarium for consultancy from Sysmex (2017–2020) and CPP‐FAP (2018), speaker's fee from Norgine Iberia, and editorial fee from Elsevier as editor of Gastroenterologia y Hepatologia. EQ and AL received an honorarium for consultancy from Sysmex (2017–2020). The other authors declare no conflict of interest regarding this study.
Figures
References
-
- National Institute for Health and Care Excellence (NICE) . Suspected cancer: recognition and referral. NICE Guideline NG12; 2017. https://www.nice.org.uk/guidance/ng12. Accessed 5 May 2022. - PubMed
-
- van Melle M, Yep Manzano SIS, Wilson H, Hamilton W, Walter FM, Bailey SER. Faecal immunochemical test to triage patients with abdominal symptoms for suspected colorectal cancer in primary care: review of international use and guidelines. Fam Pract. 2020;37(5):606–615. 10.1093/fampra/cmaa043 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical