The benefit and risk of PD-1/PD-L1 inhibitors plus anti-angiogenic agents as second or later-line treatment for patients with advanced non-small-cell lung cancer: a systematic review and single-arm meta-analysis of prospective clinical trials
- PMID: 37614237
- PMCID: PMC10442655
- DOI: 10.3389/fimmu.2023.1218258
The benefit and risk of PD-1/PD-L1 inhibitors plus anti-angiogenic agents as second or later-line treatment for patients with advanced non-small-cell lung cancer: a systematic review and single-arm meta-analysis of prospective clinical trials
Abstract
Background: Previous studies revealed that Programmed cell death protein 1 (PD-1)/Programmed cell death-Ligand protein 1 (PD-L1) inhibitors plus anti-angiogenic agents had extensive anti-tumor activities. However, almost all studies on the efficacy and safety of PD-1/PD-L1 inhibitors plus anti-angiogenic agents as second or later-line treatment for patients with advanced non-small cell lung cancer are non-randomized controlled trials with small sample sizes, which might lead to a lack of effective metrics to assess the effectiveness and safety of the therapeutic regimen. Here, this meta-analysis aimed to evaluate the efficacy and safety of PD-1/PD-L1 inhibitors plus anti-angiogenic agents as second or later-line treatment for patients with advanced non-small cell lung cancer.
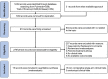
Methods: A single-arm meta-analysis was performed, and published literature from PubMed, Web of Science and Embase databases as of January 13, 2023, was systematically retrieved. We used the Cochrane risk of bias tool and methodological index for non-randomized studies (MINORS) Methodological items to evaluate the quality of eligible clinical trials. Outcomes including overall response rate (ORR), disease control rate (DCR), progression-free survival (PFS), overall survival (OS), and adverse events (AEs) were extracted for further analysis. The random effect model is used to calculate the pooled parameters.
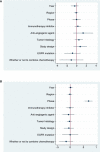
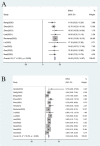
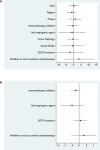
Results: 19 studies (16 were non-comparative single-arm clinical trials and 3 were randomized controlled trials) were enrolled in this meta-analysis. In terms of tumor response, the pooled ORR and DCR were 22.4% (95% CI, 16.6-28.1%) and 76.8% (95% CI, 72.6-81.1%), respectively. With regard to survival analysis, the pooled PFS and OS were 5.20 (95% CI, 4.46-5.93) months and 14.09 (95% CI, 13.20-14.97) months, respectively. The pooled grade ≥3 adverse effect (AE) rate was 47.6% (95% CI, 33.1-62.0%).
Conclusion: PD-1/PD-L1 inhibitors plus anti-angiogenic agents has promising efficacy and safety as second or later-line treatment in patients with advanced non-small cell lung cancer.
Systematic review registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023407559.
Keywords: PD-1/PD-L1 inhibitors; advanced non-small cell lung cancer; anti-angiogenic agents; meta-analysis; second or later-line therapy.
Copyright © 2023 Chen, Mo, Jiang, Zhou, Gan and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Efficacy and safety of first-line PD-1/PD-L1 inhibitor in combination with CTLA-4 inhibitor in the treatment of patients with advanced non-small cell lung cancer: a systemic review and meta-analysis.Front Immunol. 2025 Feb 6;16:1515027. doi: 10.3389/fimmu.2025.1515027. eCollection 2025. Front Immunol. 2025. PMID: 39981238 Free PMC article.
-
PD-1/PD-L1 inhibitors plus anti-angiogenic agents with or without chemotherapy versus PD-1/PD-L1 inhibitors plus chemotherapy as second or later-line treatment for patients with advanced non-small cell lung cancer: A real-world retrospective cohort study.Front Immunol. 2022 Dec 7;13:1059995. doi: 10.3389/fimmu.2022.1059995. eCollection 2022. Front Immunol. 2022. PMID: 36569915 Free PMC article.
-
Efficacy and safety of PD-1 inhibitors as second-line treatment for advanced squamous esophageal cancer: a systematic review and network meta-analysis with a focus on PD-L1 expression levels.Front Immunol. 2025 Jan 23;15:1510145. doi: 10.3389/fimmu.2024.1510145. eCollection 2024. Front Immunol. 2025. PMID: 39916953 Free PMC article.
-
Comparative efficacy and safety of PD-1/PD-L1 Inhibitors versus platinum-based chemotherapy for the first-line treatment of advanced non-small cell lung cancer: a meta analysis of randomized controlled trials.Pharmacol Res. 2020 Oct;160:105194. doi: 10.1016/j.phrs.2020.105194. Epub 2020 Sep 13. Pharmacol Res. 2020. PMID: 32937178
-
Efficacy and Safety of PD-1/PD-L1 Inhibitors in Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-analysis.Adv Ther. 2023 Feb;40(2):521-549. doi: 10.1007/s12325-022-02371-3. Epub 2022 Nov 18. Adv Ther. 2023. PMID: 36399316
Cited by
-
A meta-analysis of the efficacy of programmed cell death 1/its ligand inhibitors plus cytotoxic T-lymphocyte-associated antigen 4 inhibitors in non-small cell lung cancer.Front Pharmacol. 2024 Feb 5;15:1267763. doi: 10.3389/fphar.2024.1267763. eCollection 2024. Front Pharmacol. 2024. PMID: 38379896 Free PMC article.
-
Mapping the research trends and hotspots on solid pulmonary nodules from 2000-2024: insights from bibliometric analysis.J Thorac Dis. 2025 Jun 30;17(6):3498-3515. doi: 10.21037/jtd-24-1669. Epub 2025 Jun 11. J Thorac Dis. 2025. PMID: 40688341 Free PMC article.
-
Evaluation of the effectiveness and safety of combining PD-1/PD-L1 inhibitors with anti-angiogenic agents in unresectable hepatocellular carcinoma: a systematic review and meta-analysis.Front Immunol. 2024 Sep 17;15:1468440. doi: 10.3389/fimmu.2024.1468440. eCollection 2024. Front Immunol. 2024. PMID: 39355241 Free PMC article.
-
The Predictive Significance of Various Prognostic Scoring Systems on the Efficacy of Immunotherapy in Non-Small Cell Lung Cancer Patients: A Retrospective Study.Health Sci Rep. 2025 Apr 21;8(4):e70713. doi: 10.1002/hsr2.70713. eCollection 2025 Apr. Health Sci Rep. 2025. PMID: 40260026 Free PMC article.
-
Combination of immune checkpoint inhibitors with multi-targeted tyrosine kinase inhibitors for second- or later-line therapy of non-small cell lung cancer: a systematic review and meta-analysis.Transl Lung Cancer Res. 2025 May 30;14(5):1724-1739. doi: 10.21037/tlcr-2024-1204. Epub 2025 May 28. Transl Lung Cancer Res. 2025. PMID: 40535065 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

