Cardiovascular safety of Janus kinase inhibitors in patients with rheumatoid arthritis: systematic review and network meta-analysis
- PMID: 37614310
- PMCID: PMC10442954
- DOI: 10.3389/fphar.2023.1237234
Cardiovascular safety of Janus kinase inhibitors in patients with rheumatoid arthritis: systematic review and network meta-analysis
Abstract
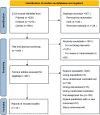
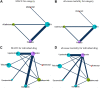
Background: Janus kinase (JAK) inhibitors have emerged as a progressively utilized therapeutic approach for the management of rheumatoid arthritis (RA). However, the complete determination of their cardiovascular safety remains inconclusive. Hence, the primary objective of this network meta-analysis is to meticulously assess and juxtapose the cardiovascular risks linked to distinct JAK inhibitors employed in RA patients. Methods: A systematic review and network meta-analysis were meticulously conducted, encompassing a collection of randomized controlled trials (RCTs) that focused on investigating the incidence of major adverse cardiovascular events (MACE) and all-cause mortality associated with Janus kinase (JAK) inhibitors administered to patients with rheumatoid arthritis (RA). Extensive exploration was performed across multiple electronic databases, incorporating studies published until March 2023. To be included in this analysis, the RCTs were required to involve adult participants diagnosed with RA who received treatment with JAK inhibitors. To ensure accuracy, two authors independently undertook the selection of eligible RCTs and meticulously extracted aggregate data. In order to examine the outcomes of MACE and all-cause mortality, a frequentist graph theoretical approach within network meta-analyses was employed, utilizing random-effects models. Third study has been registered on PROSPERO under the reference CRD42022384611. Findings: A specific selection encompassing a total of 14 meticulously chosen randomized controlled trials was undertaken, wherein 13,524 patients were assigned randomly to distinct treatment interventions. The analysis revealed no notable disparity in the occurrence of major adverse cardiovascular events (MACE) between the interventions and the placebo group. However, in comparison to adalimumab, the employment of JAK inhibitors exhibited an association with higher rates of all-cause mortality [odds ratio (OR): 1.7, 95% confidence interval (CI): 1.02-2.81]. This observed increase in risk primarily stemmed from the usage of tofacitinib (OR: 1.9, 95% CI: 1.12-3.23). None of the other JAK inhibitors exhibited a statistically significant variance in all-cause mortality when compared to adalimumab. Interpretation: Our study suggests that JAK inhibitors may not increase the risk of MACE in RA patients but may be associated with a higher risk of all-cause mortality compared to adalimumab, primarily due to tofacitinib use. Rheumatologists should carefully consider the cardiovascular risks when prescribing JAK inhibitors, particularly tofacitinib, for RA patients. Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=384611, CRD42022384611.
Keywords: Janus kinase inhbitors; all-cause mortality; cardiovascular safety; major cardiovascular adverse events; network meta-analysis; rheumathoid arthritis.
Copyright © 2023 Wei, Wang, Zhao, Luo, Wang, Zhu, Su and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Comparative efficacy of five approved Janus kinase inhibitors as monotherapy and combination therapy in patients with moderate-to-severe active rheumatoid arthritis: a systematic review and network meta-analysis of randomized controlled trials.Front Pharmacol. 2024 Apr 24;15:1387585. doi: 10.3389/fphar.2024.1387585. eCollection 2024. Front Pharmacol. 2024. PMID: 38725657 Free PMC article. Review.
-
Cardiovascular and Venous Thromboembolic Risk With JAK Inhibitors in Immune-Mediated Inflammatory Skin Diseases: A Systematic Review and Meta-Analysis.JAMA Dermatol. 2024 Jan 1;160(1):28-36. doi: 10.1001/jamadermatol.2023.4090. JAMA Dermatol. 2024. PMID: 37910098 Free PMC article.
-
Oral Janus kinase inhibitors for maintenance of remission in ulcerative colitis.Cochrane Database Syst Rev. 2020 Jan 27;1(1):CD012381. doi: 10.1002/14651858.CD012381.pub2. Cochrane Database Syst Rev. 2020. PMID: 31984480 Free PMC article.
-
Risk of Major Adverse Cardiovascular Events and Venous Thromboembolism with JAK Inhibitors versus TNF Inhibitors in Rheumatoid Arthritis Patients: A Systematic Review and Meta-Analysis.Mediterr J Rheumatol. 2024 Mar 30;35(Suppl 1):10-19. doi: 10.31138/mjr.171023.rof. eCollection 2024 Mar. Mediterr J Rheumatol. 2024. PMID: 38756933 Free PMC article.
-
The Efficacy and Safety of Janus Kinase Inhibitors for Patients With COVID-19: A Living Systematic Review and Meta-Analysis.Front Med (Lausanne). 2022 Jan 27;8:800492. doi: 10.3389/fmed.2021.800492. eCollection 2021. Front Med (Lausanne). 2022. PMID: 35155477 Free PMC article.
Cited by
-
Kinase Inhibitors FDA Approved 2018-2023: Drug Targets, Metabolic Pathways, and Drug-Induced Toxicities.Drug Metab Dispos. 2024 May 16;52(6):479-492. doi: 10.1124/dmd.123.001430. Drug Metab Dispos. 2024. PMID: 38286637 Free PMC article. Review.
-
Safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral allosteric TYK2 inhibitor ESK-001 using a randomized, double-blind, placebo-controlled study design.Clin Transl Sci. 2024 Dec;17(12):e70094. doi: 10.1111/cts.70094. Clin Transl Sci. 2024. PMID: 39604226 Free PMC article. Clinical Trial.
-
Serum Lipids Alterations in Patients Under Systemic JAK Inhibitors Treatments in Dermatology: Clinical Aspects and Management.Medicina (Kaunas). 2025 Jan 1;61(1):54. doi: 10.3390/medicina61010054. Medicina (Kaunas). 2025. PMID: 39859036 Free PMC article. Review.
-
JAK inhibitor withdrawal causes a transient pro-inflammatory cascade: A potential mechanism for major adverse cardiac events.PLoS One. 2025 Jun 16;20(6):e0311706. doi: 10.1371/journal.pone.0311706. eCollection 2025. PLoS One. 2025. PMID: 40522928 Free PMC article.
-
Metabolic syndrome and psoriatic arthritis: the role of weight loss as a disease-modifying therapy.Ther Adv Musculoskelet Dis. 2024 Aug 19;16:1759720X241271886. doi: 10.1177/1759720X241271886. eCollection 2024. Ther Adv Musculoskelet Dis. 2024. PMID: 39161788 Free PMC article. Review.
References
-
- Aletaha D., Neogi T., Silman A. J., Funovits J., Felson D. T., Bingham C. O., 3rd, et al. (2010). 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588. 10.1136/ard.2010.138461 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

