Comparative cryopreservation of bovine and porcine primary hepatocytes
- PMID: 37614462
- PMCID: PMC10442649
- DOI: 10.3389/fvets.2023.1211135
Comparative cryopreservation of bovine and porcine primary hepatocytes
Abstract
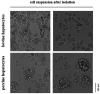
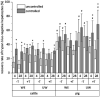
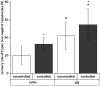
The isolation of primary hepatocytes from liver tissue of farm animals yields a very high number of cells, and a part of them can be stored by cryopreservation for future experiments. As no experience exists with the cryopreservation of hepatocytes from cattle, our study aimed at the cryopreservation of bovine hepatocytes by use of different protocols compared with the cryopreservation of hepatocytes from pig. We tested different freezing media (William's Medium E vs. University of Wisconsin solution), cryoprotectants (dimethyl sulfoxide with vs. without trehalose as additional additive), freezing systems (standard freezing container vs. controlled-rate freezer) and freezing times (4 vs. 28 d). These tests identified a general influence of species and freezing systems, whereas the influence of freezing media, trehalose additive and freezing time was less or not obvious. In this regard, we determined a mean recovery of 30% of bovine hepatocytes and 55% of porcine hepatocytes cryopreserved in a controlled-rate freezer, whereas the rates were about 10% less when hepatocytes were frozen in a standard freezing container. In accordance with this observation, the cultivation of cryopreserved hepatocytes from cattle was less effective than that of porcine hepatocytes. Hepatocytes from cattle can be successfully cryopreserved and partially cultured after cryopreservation but with lower percentage than porcine hepatocytes.
Keywords: cattle; controlled-rate freezer; freezing medium; liver; pig; primary cells; trehalose.
Copyright © 2023 Andres, Bartling, Stiensmeier, Starke and Schmicke.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Cryopreservation of porcine spermatogonial stem cells by slow-freezing testis tissue in trehalose.J Anim Sci. 2014 Mar;92(3):984-95. doi: 10.2527/jas.2013-6843. Epub 2014 Feb 6. J Anim Sci. 2014. PMID: 24504041
-
Freezing of dendritic cells with trehalose as an additive in the conventional freezing medium results in improved recovery after cryopreservation.Transfusion. 2019 Feb;59(2):686-696. doi: 10.1111/trf.15028. Epub 2018 Nov 20. Transfusion. 2019. PMID: 30456902
-
Improvement of hepatocyte viability after cryopreservation by supplementation of long-chain oligosaccharide in the freezing medium in rats and humans.Cell Transplant. 2006;15(10):911-9. doi: 10.3727/000000006783981404. Cell Transplant. 2006. PMID: 17299996
-
Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction.Drug Metab Rev. 2000 Feb;32(1):81-118. doi: 10.1081/dmr-100100564. Drug Metab Rev. 2000. PMID: 10711408 Review.
-
Cryopreservation of isolated human hepatocytes for transplantation: State of the art.Cryobiology. 2006 Oct;53(2):149-59. doi: 10.1016/j.cryobiol.2006.05.004. Epub 2006 Jun 21. Cryobiology. 2006. PMID: 16793034 Review.
Cited by
-
Effect of low-frequency assisted ultrasonic on cryopreservation of L-02 hepatocyte cells.Front Cell Dev Biol. 2025 Apr 28;13:1571198. doi: 10.3389/fcell.2025.1571198. eCollection 2025. Front Cell Dev Biol. 2025. PMID: 40356599 Free PMC article.
References
-
- Gebhardt R, Hengstler JG, Muller D, Glockner R, Buenning P, Laube B, et al. . New hepatocyte in vitro systems for drug metabolism: metabolic capacity and recommendations for application in basic research and drug development, standard operation procedures. Drug Metab Rev. (2003) 35:145–213. doi: 10.1081/dmr-120023684, PMID: - DOI - PubMed
-
- Gerbal-Chaloin S, Briolotti P, Daujat-Chavanieu M, Rasmussen MK. Primary hepatocytes isolated from human and porcine donors display similar patterns of cytochrome p450 expression following exposure to prototypical activators of AhR, CAR and PXR. Curr Res Toxicol. (2021) 2:149–58. doi: 10.1016/j.crtox.2021.03.002, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

