Echocardiographic Imaging in Transcatheter Structural Intervention: An AAE Review Paper
- PMID: 37614546
- PMCID: PMC10442887
- DOI: 10.1016/j.jacasi.2023.05.012
Echocardiographic Imaging in Transcatheter Structural Intervention: An AAE Review Paper
Abstract
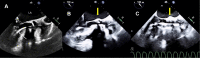
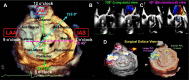
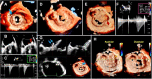
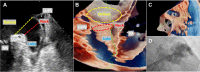
Transcatheter structural heart intervention (TSHI) has gained popularity over the past decade as a means of cardiac intervention in patients with prohibitive surgical risks. Following the exponential rise in cases and devices developed over the period, there has been increased focus on developing the role of "structural imagers" amongst cardiologists. This review, as part of a growing initiative to develop the field of interventional echocardiography, aims to highlight the role of echocardiography in myriad TSHIs available within Asia. We first discuss the various echocardiography-based imaging modalities, including 3-dimensional echocardiography, fusion imaging, and intracardiac echocardiography. We then highlight a selected list of structural interventions available in the region-a combination of established interventions alongside novel approaches-describing key anatomic and pathologic characteristics related to the relevant structural heart diseases, before delving into various aspects of echocardiography imaging for each TSHI.
Keywords: echocardiography; interventional echocardiography; review; transcatheter structural intervention.
© 2023 The Authors.
Conflict of interest statement
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
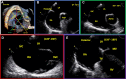
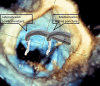
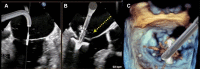
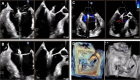
Figures

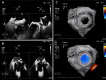
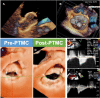

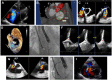
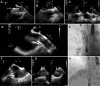
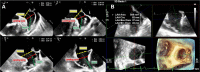
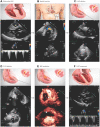
References
-
- Hamid N., Ewe S.H. Interventional echocardiography: current role and progress. Proc Singap Healthc. 2015;24(1):4–15.
-
- Ludman P.F., Moat N., de Belder M.A., et al. Transcatheter aortic valve implantation in the United Kingdom: temporal trends, predictors of outcome, and 6-year follow-up: a report from the UK Transcatheter Aortic Valve Implantation (TAVI) registry, 2007 to 2012. Circulation. 2015;131(13):1181–1190. - PubMed
-
- Durko A.P., Osnabrugge R.L., Van Mieghem N.M., et al. Annual number of candidates for transcatheter aortic valve implantation per country: current estimates and future projections. Eur Heart J. 2018;39(28):2635–2642. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

