Classification of genotypes based on the VP1 gene of feline calicivirus and study of cross-protection between different genotypes
- PMID: 37614595
- PMCID: PMC10442547
- DOI: 10.3389/fmicb.2023.1226877
Classification of genotypes based on the VP1 gene of feline calicivirus and study of cross-protection between different genotypes
Abstract
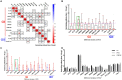
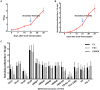
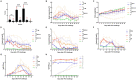
Feline calicivirus (FCV) causes upper respiratory tract diseases and even death in cats, thereby acting as a great threat to feline animals. Currently, FCV prevention is mainly achieved through vaccination, but the effectiveness of vaccination is limited. In this study, 105 FCV strain VP1 sequences with clear backgrounds were downloaded from the NCBI and subjected to a maximum likelihood method for systematic evolutionary analysis. Based on the genetic analysis results, FCV-positive sera were prepared using SPF mice and Chinese field cats as target animals, followed by a cross-neutralization assay conducted on the different genotype strains and in vivo challenge tests were carried out to further verify with the strain with best cross-protection effect. The results revealed that FCV was mainly divided into two genotypes: GI and GII. The GI genotype strains are prevalent worldwide, but all GII genotype strains were isolated from Asia, indicating a clear geographical feature. This may form resistance to FCV prevention in Asia. The in vitro neutralization assay conducted using murine serum demonstrated that the cross-protection effect varied among strains. A strain with broad-spectrum neutralization properties, DL39, was screened. This strain could produce neutralizing titers (10 × 23.08-10 × 20.25) against all strains used in this study. The antibody titers against the GI strains were 10 × 23.08-10 × 20.5 and those against the GII strains were 10 × 20.75-10 × 20.25. Preliminary evidence suggested that the antibody titer of the DL39 strain against GI was higher than that against GII. Subsequent cross-neutralization assays with cat serum prepared with the DL39 strain and each strain simultaneously yielded results similar to those described above. In vivo challenge tests revealed that the DL39 strain-immunized cats outperformed the positive controls in all measures. The results of several trials demonstrated that strain DL39 can potentially be used as a vaccine strain. The study attempted to combine the genetic diversity and phylogenetic analysis of FCV with the discovery of potential vaccines, which is crucial for developing highly effective FCV vaccines.
Keywords: FCV; VP1; broad-spectrum neutralization; challenge tests; cross-neutralization assay; genotype.
Copyright © 2023 Yang, Liu, Chen, Feng, Qi, Zheng, Wang, Kang, Jiang, Yang, Qu and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification and Pathogenicity Analysis of Feline Calicivirus in Shanghai and Guangdong, China.Transbound Emerg Dis. 2025 Jun 4;2025:8729295. doi: 10.1155/tbed/8729295. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40503218 Free PMC article.
-
An effectively protective VLP vaccine candidate for both genotypes of feline calicivirus.Front Immunol. 2024 Dec 20;15:1515342. doi: 10.3389/fimmu.2024.1515342. eCollection 2024. Front Immunol. 2024. PMID: 39759504 Free PMC article.
-
Screening and Immune Efficacy Evaluation of Antigens with Protection Against Feline Calicivirus.Vaccines (Basel). 2024 Oct 24;12(11):1205. doi: 10.3390/vaccines12111205. Vaccines (Basel). 2024. PMID: 39591108 Free PMC article.
-
An Update on Feline Calicivirus.Schweiz Arch Tierheilkd. 2022 Mar;164(3):225-241. doi: 10.17236/sat00346. Schweiz Arch Tierheilkd. 2022. PMID: 35232714 Review. English.
-
Feline calicivirus.Vet Res. 2007 Mar-Apr;38(2):319-35. doi: 10.1051/vetres:2006056. Epub 2007 Feb 13. Vet Res. 2007. PMID: 17296159 Review.
Cited by
-
Whole-genome sequencing of feline calicivirus in domestic cats, South Korea, 2023.Front Vet Sci. 2025 Apr 28;12:1570761. doi: 10.3389/fvets.2025.1570761. eCollection 2025. Front Vet Sci. 2025. PMID: 40357189 Free PMC article. No abstract available.
-
A novel replication-deficient FCV vaccine provides strong immune protection in cats.J Virol. 2025 Aug 19;99(8):e0009325. doi: 10.1128/jvi.00093-25. Epub 2025 Jul 8. J Virol. 2025. PMID: 40626663 Free PMC article.
-
Identification and Pathogenicity Analysis of Feline Calicivirus in Shanghai and Guangdong, China.Transbound Emerg Dis. 2025 Jun 4;2025:8729295. doi: 10.1155/tbed/8729295. eCollection 2025. Transbound Emerg Dis. 2025. PMID: 40503218 Free PMC article.
-
Unlocking the secrets of Feline calicivirus: advances in structural and nonstructural proteins and its role as a key model for other Caliciviruses.Virol J. 2025 May 21;22(1):152. doi: 10.1186/s12985-025-02750-6. Virol J. 2025. PMID: 40399981 Free PMC article. Review.
-
Characterization and immunogenic evaluation of feline calicivirus epidemic strains.J Vet Sci. 2025 Jul;26(4):e38. doi: 10.4142/jvs.24331. J Vet Sci. 2025. PMID: 40765225 Free PMC article.
References
-
- Afonso M. M., Pinchbeck G. L., Smith S. L., Daly J. M., Gaskell R. M., Dawson S., et al. . (2017). A multi-national European cross-sectional study of feline calicivirus epidemiology, diversity and vaccine cross-reactivity. Vaccine 35, 2753–2760. doi: 10.1016/j.vaccine.2017.03.030, PMID: - DOI - PubMed
-
- Bordicchia M., Fumian T. M., Van Brussel K., Russo A. G., Carrai M., Le S. J., et al. . (2021). Feline Calicivirus virulent systemic disease: clinical epidemiology, analysis of viral isolates and in vitro efficacy of novel antivirals in Australian outbreaks. Viruses 13:2040. doi: 10.3390/v13102040, PMID: - DOI - PMC - PubMed
-
- Brunet S., Sigoillot-Claude C., Pialot D., Poulet H. (2019). Multiple correspondence analysis on amino acid properties within the variable region of the capsid protein shows differences between classical and virulent systemic feline Calicivirus strains. Viruses 11:1090. doi: 10.3390/v11121090, PMID: - DOI - PMC - PubMed
-
- Coyne K. P., Dawson S., Radford A. D., Cripps P. J., Porter C. J., McCracken C. M., et al. . (2006). Long-term analysis of feline calicivirus prevalence and viral shedding patterns in naturally infected colonies of domestic cats. Vet. Microbiol. 118, 12–25. doi: 10.1016/j.vetmic.2006.06.026, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

