Biofabrication of functional bone tissue: defining tissue-engineered scaffolds from nature
- PMID: 37614632
- PMCID: PMC10444209
- DOI: 10.3389/fbioe.2023.1185841
Biofabrication of functional bone tissue: defining tissue-engineered scaffolds from nature
Abstract
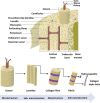
Damage to bone leads to pain and loss of movement in the musculoskeletal system. Although bone can regenerate, sometimes it is damaged beyond its innate capacity. Research interest is increasingly turning to tissue engineering (TE) processes to provide a clinical solution for bone defects. Despite the increasing biomimicry of tissue-engineered scaffolds, significant gaps remain in creating the complex bone substitutes, which include the biochemical and physical conditions required to recapitulate bone cells' natural growth, differentiation and maturation. Combining advanced biomaterials with new additive manufacturing technologies allows the development of 3D tissue, capable of forming cell aggregates and organoids based on natural and stimulated cues. Here, we provide an overview of the structure and mechanical properties of natural bone, the role of bone cells, the remodelling process, cytokines and signalling pathways, causes of bone defects and typical treatments and new TE strategies. We highlight processes of selecting biomaterials, cells and growth factors. Finally, we discuss innovative tissue-engineered models that have physiological and anatomical relevance for cancer treatments, injectable stimuli gels, and other therapeutic drug delivery systems. We also review current challenges and prospects of bone TE. Overall, this review serves as guide to understand and develop better tissue-engineered bone designs.
Keywords: 3D bioprinting; biofabrication; biomaterials; bone tissue; growth factor; hydrogel; organoid; tissue engineering and regenerative medicine.
Copyright © 2023 Rifai, Weerasinghe, Tilaye, Nisbet, Hodge, Pasco, Williams, Samarasinghe and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
-
- Alvarez K., Nakajima H. (2009). Metallic scaffolds for bone regeneration. Mater. (Basel) 2 (3), 790–832. 10.3390/ma2030790 - DOI
Publication types
LinkOut - more resources
Full Text Sources

