Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a real-life multicenter study-IL PSO (Italian landscape psoriasis)
- PMID: 37614958
- PMCID: PMC10442506
- DOI: 10.3389/fmed.2023.1243843
Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a real-life multicenter study-IL PSO (Italian landscape psoriasis)
Abstract
Introduction: Bimekizumab is a monoclonal antibody that targets Interleukin-17 A and F, approved for the treatment of moderate-to-severe plaque psoriasis. While bimekizumab has been evaluated in several phase-III clinical trials, real-world evidence is still very limited.
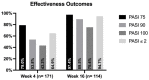
Method: This multicenter retrospective study included patients affected by plaque psoriasis treated with bimekizumab from May 1, 2022 to April 30, 2023, at 19 Italian referral hospitals. Patients affected by moderate-to-severe plaque psoriasis eligible for systemic treatments were included. The effectiveness of bimekizumab was evaluated in terms of reduction in psoriasis area and severity index (PASI) compared with baseline at weeks 4 and 16. The main outcomes were the percentages of patients achieving an improvement of at least 75% (PASI75), 90% (PASI90) and 100% (PASI100) in PASI score.
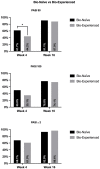
Results: The study included 237 patients who received at least one injection of bimekizumab. One hundred and seventy-one patients and 114 reached four and 16 weeks of follow-up, respectively. Complete skin clearance was achieved by 43.3% and 75.4% of patients at weeks 4 and 16, respectively. At week 16, 86.8% of patients reported no impact on their quality of life. At week 16, there were no significant differences between bio-naïve and bio-experienced patients in terms of PASI75, PASI90 and PASI100. The most commonly reported adverse events (AEs) were oral candidiasis (10.1%). No severe AEs or AEs leading to discontinuation were observed throughout the study.
Conclusion: Our experience supports the effectiveness and tolerability of bimekizumab in a real-world setting with similar results compared with phase-III clinical trials.
Keywords: bimekizumab; biologics; psoriasis; psoriasis treatment; real-life.
Copyright © 2023 Gargiulo, Narcisi, Ibba, Balato, Bianchi, Brianti, Buononato, Burlando, Caldarola, Campanati, Campione, Carrera, Carugno, Cristaudo, Cusano, Dapavo, Dattola, De Simone, Gaiani, Gisondi, Giunta, Loconsole, Maione, Mortato, Marzano, Maurelli, Megna, Mercuri, Offidani, Orsini, Parodi, Pellacani, Potestio, Quaglino, Richetta, Romano, Sena, Venturini, Malagoli and Costanzo.
Conflict of interest statement
LG has been a consultant for Almirall. PM has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. AB has received honoraria for participation in advisory boards, meetings, or as speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. LB has received honoraria as a speaker or consultant for AbbVie, Janssen, Almirall Eli-Lilly, Leopharma, Novartis, Sanofi, Pfizer, and UCB Pharma. GC reports consulting fees or honorarium and payment for lectures from Lilly and Novartis. CD reports consulting fees or honorarium from Abbvie, Amgen, Novartis, Celgene, Sanofi, UCB Pharma, Janssen, and Lilly and payment for lectures from Abbvie, Lilly, Novartis, UCB Pharma, and Celgene. PG has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Leo-Pharma, Eli-Lilly, Novartis, Pierre Febre, Sandoz, Sanofi, and UCB. MB has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, and UCB Pharma. CC has served as a board participant or speaker for Abbvie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. PD has been a speaker for Novartis, Abbvie, Sanofi, UCB, Janssen, Lilly, and LeoPharma. FG acted as a speaker or consultant for Novartis, Abbvie, Eli Lilly, Celgene, LeoPharma, and Almirall. AG received grants or is an investigator for Biogen and Lilly; and is a consultant/advisory board/speaker for AbbVie, Almirall, Celgene, Janssen, Leo Pharma, Eli Lilly, Merck Sharpe Dohme, Novartis, Pfizer, Sandoz, and UCB. FL served on advisory boards and/or received honoraria for lectures from Abbvie, Janssen-Cilag, Novartis, Lilly, Sanofi. AM reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi, and UCB. MMe acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo-Pharma, UCB, and Novartis. AO acted as a speaker and consultant for Abbvie, Eli Lilly, Novartis, Celgene, Sanofi, Galderma, Leo Pharma, and Pierre Fabre. MV served as a speaker or advisory board member for Abbvie, Almirall, Amgen, Eli-Lilly, Galderma, Leo Pharma, Novartis, Pierre Fabre, and UCB Pharma. ACo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. AN has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen, and Boehringer Ingelheim. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Gordon KB, Foley P, Krueger JG, Pinter A, Reich K, Vender R, et al. . Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (be ready): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. (2021) 397:475–86. doi: 10.1016/S0140-6736(21)00126-4 - DOI - PubMed
-
- Reich K, Papp KA, Blauvelt A, Langley RG, Armstrong A, Warren RB, et al. . Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (be vivid): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. (2021) 397:487–98. doi: 10.1016/S0140-6736(21)00125-2, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous