A novel role of RNase L in the development of nonalcoholic steatohepatitis
- PMID: 37615181
- PMCID: PMC10715709
- DOI: 10.1096/fj.202300621R
A novel role of RNase L in the development of nonalcoholic steatohepatitis
Abstract
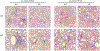
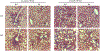
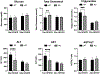
Nonalcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and affects about 25% of the population globally. NAFLD has the potential to cause significant liver damage in many patients because it can progress to nonalcoholic steatohepatitis (NASH) and cirrhosis, which substantially increases disease morbidity and mortality. Despite the key role of innate immunity in the disease progression, the underlying molecular and pathogenic mechanisms remain to be elucidated. RNase L is a key enzyme in interferon action against viral infection and displays pleiotropic biological functions such as control of cell proliferation, apoptosis, and autophagy. Recent studies have demonstrated that RNase L is involved in innate immunity. In this study, we revealed that RNase L contributed to the development of NAFLD, which further progressed to NASH in a time-dependent fashion after RNase L wild-type (WT) and knockout mice were fed with a high-fat and high-cholesterol diet. RNase L WT mice showed significantly more severe NASH, evidenced by widespread macro-vesicular steatosis, hepatocyte ballooning degeneration, inflammation, and fibrosis, although physiological and biochemical data indicated that both types of mice developed obesity, hyperglycemia, hypercholesterolemia, dysfunction of the liver, and systemic inflammation at different extents. Further investigation demonstrated that RNase L was responsible for the expression of some key genes in lipid metabolism, inflammation, and fibrosis signaling. Taken together, our results suggest that a novel therapeutic intervention for NAFLD may be developed based on regulating the expression and activity of RNase L.
Keywords: HFHCD; NAFLD; NASH; RNase L; mouse.
© 2023 The Authors. The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
Figures





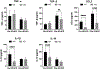
Similar articles
-
Disruption of hepatic small heterodimer partner induces dissociation of steatosis and inflammation in experimental nonalcoholic steatohepatitis.J Biol Chem. 2020 Jan 24;295(4):994-1008. doi: 10.1074/jbc.RA119.010233. Epub 2019 Dec 12. J Biol Chem. 2020. PMID: 31831621 Free PMC article.
-
Bax inhibitor-1 protects from nonalcoholic steatohepatitis by limiting inositol-requiring enzyme 1 alpha signaling in mice.Hepatology. 2018 Aug;68(2):515-532. doi: 10.1002/hep.29847. Epub 2018 May 18. Hepatology. 2018. PMID: 29457838
-
Knockout of sulfatase 2 is associated with decreased steatohepatitis and fibrosis in a mouse model of nonalcoholic fatty liver disease.Am J Physiol Gastrointest Liver Physiol. 2020 Sep 1;319(3):G333-G344. doi: 10.1152/ajpgi.00150.2019. Epub 2020 Jul 20. Am J Physiol Gastrointest Liver Physiol. 2020. PMID: 32683952 Free PMC article.
-
Potential Therapeutic Application of Estrogen in Gender Disparity of Nonalcoholic Fatty Liver Disease/Nonalcoholic Steatohepatitis.Cells. 2019 Oct 15;8(10):1259. doi: 10.3390/cells8101259. Cells. 2019. PMID: 31619023 Free PMC article. Review.
-
Histopathology of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis.World J Gastroenterol. 2014 Nov 14;20(42):15539-48. doi: 10.3748/wjg.v20.i42.15539. World J Gastroenterol. 2014. PMID: 25400438 Free PMC article. Review.
References
-
- Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM; Sanyal AJ. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018; 67:328–357. - PubMed
-
- Younossi Z, Tacke F, Arrese M, Chander Sharma B, Mostafa I, Bugianesi E, Wai-Sun Wong V, Yilmaz Y, George J, Fan J., et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019; 69:2672–2682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

