Severe acute respiratory syndrome coronavirus 2 seroprevalence and longitudinal antibody response following natural infection in pregnancy: A prospective cohort study
- PMID: 37615311
- PMCID: PMC10467162
- DOI: 10.1177/17455057231190955
Severe acute respiratory syndrome coronavirus 2 seroprevalence and longitudinal antibody response following natural infection in pregnancy: A prospective cohort study
Abstract
Background: Antenatal care provides unique opportunities to assess severe acute respiratory syndrome coronavirus 2 seroprevalence and antibody response duration after natural infection detected during pregnancy; transplacental antibody transfer may inform peripartum and neonatal protection. We estimated seroprevalence and durability of antibodies from natural infection (anti-nucleocapsid immunoglobulin G) among pregnant people, and evaluated transplacental transfer efficiency.
Objective and design: We conducted a cross-sectional study to measure severe acute respiratory syndrome coronavirus 2 seroprevalence, and a prospective cohort study to longitudinally measure anti-nucleocapsid immunoglobulin G responses and transplacental transfer of maternally derived anti-nucleocapsid antibodies.
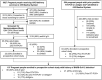
Methods: We screened pregnant people for the seroprevalence study between 9 December 2020 and 19 June 2021 for anti-nucleocapsid immunoglobulin G in Seattle, Washington. We enrolled anti-nucleocapsid immunoglobulin G positive people from the seroprevalence study or identified through medical records with positive reverse transcription polymerase chain reaction or antigen positive results in a prospective cohort between 9 December 2020 and 9 August 2022.
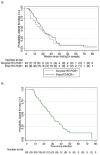
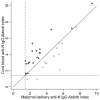
Results: In the cross-sectional study (N = 1284), 5% (N = 65) tested severe acute respiratory syndrome coronavirus 2 anti-nucleocapsid immunoglobulin G positive, including 39 (60%) without prior positive reverse transcription polymerase chain reaction results and 42 (65%) without symptoms. In the prospective cohort study (N = 107 total; N = 65 from the seroprevalence study), 86 (N = 80%) had anti-nucleocapsid immunoglobulin G positive results during pregnancy. Among 63 participants with delivery samples and prior anti-nucleocapsid positive results, 29 (46%) were anti-nucleocapsid immunoglobulin G negative by delivery. Of 34 remaining anti-nucleocapsid immunoglobulin G positive at delivery with paired cord blood, 19 (56%) had efficient transplacental anti-nucleocapsid immunoglobulin G antibody transfer. Median time from first anti-nucleocapsid immunoglobulin G positive to below positive antibody threshold was 19 weeks and did not differ by prior positive reverse transcription polymerase chain reaction status.
Conclusions: Maternally derived severe acute respiratory syndrome coronavirus 2 antibodies to natural infection may wane before delivery. Vaccines are recommended for pregnant persons to reduce severe illness and confer protection to infants.
Keywords: SARS-CoV-2; antibody; natural infection; pregnancy; seroprevalence; transplacental transfer.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JAE reports serving as a consultant for Sanofi Pasteur, AstraZeneca, Meissa Vaccines, Pfizer, and Moderna and has received grant support from Pfizer, GlaxoSmithKline, AstraZeneca, Moderna and Merck. ABK reports serving as an unpaid consultant for GlaxoSmith-Kline and as a consultant for Pfizer and has received grant support from Pfizer and Merck. ALD, JNE, MCA, EAW, BAR, ALG and SML received grant support from Merck.
Figures

Similar articles
-
Assessment of Maternal and Neonatal SARS-CoV-2 Viral Load, Transplacental Antibody Transfer, and Placental Pathology in Pregnancies During the COVID-19 Pandemic.JAMA Netw Open. 2020 Dec 1;3(12):e2030455. doi: 10.1001/jamanetworkopen.2020.30455. JAMA Netw Open. 2020. PMID: 33351086 Free PMC article.
-
Relationship between viral load, infection-to-delivery interval and mother-to-child transfer of anti-SARS-CoV-2 antibodies.Ultrasound Obstet Gynecol. 2021 Jun;57(6):974-978. doi: 10.1002/uog.23639. Ultrasound Obstet Gynecol. 2021. PMID: 33798280 Free PMC article.
-
Maternal Antibody Response, Neutralizing Potency, and Placental Antibody Transfer After Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infection.Obstet Gynecol. 2021 Aug 1;138(2):189-197. doi: 10.1097/AOG.0000000000004440. Obstet Gynecol. 2021. PMID: 33910220 Free PMC article.
-
The Duration, Dynamics, and Determinants of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Antibody Responses in Individual Healthcare Workers.Clin Infect Dis. 2021 Aug 2;73(3):e699-e709. doi: 10.1093/cid/ciab004. Clin Infect Dis. 2021. PMID: 33400782 Free PMC article.
-
Timing of SARS-CoV-2 vaccination during the third trimester of pregnancy and transplacental antibody transfer: a prospective cohort study.Clin Microbiol Infect. 2022 Mar;28(3):419-425. doi: 10.1016/j.cmi.2021.10.003. Epub 2021 Nov 3. Clin Microbiol Infect. 2022. PMID: 34740773 Free PMC article.
Cited by
-
Factors modulating maternofetal transfer of IgG antibodies following SARS-CoV-2 gestational infection.Rev Inst Med Trop Sao Paulo. 2025 Apr 14;67:e29. doi: 10.1590/S1678-9946202567029. eCollection 2025. Rev Inst Med Trop Sao Paulo. 2025. PMID: 40243801 Free PMC article.
-
Hybrid Immunity to SARS-CoV-2 During Pregnancy Provides More Durable Infant Antibody Responses Compared to Natural Infection Alone.J Infect Dis. 2024 Jun 14;229(6):1728-1739. doi: 10.1093/infdis/jiad592. J Infect Dis. 2024. PMID: 38128542 Free PMC article.
References
-
- Centers for Disease Control and Prevention. COVID-19 vaccination for pregnant people to prevent serious illness, deaths, and adverse pregnancy outcomes from COVID-19. Health Alert Network (HAN), 2021, https://emergency.cdc.gov/han/2021/han00453.asp
-
- Centers for Disease Control and Prevention. COVID-19 vaccines while pregnant or breastfeeding, https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregn... (accessed 13 June 2022). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

