Understanding Reactive Sulfur Species through P/S Synergy
- PMID: 37615644
- PMCID: PMC11131337
- DOI: 10.1021/acs.inorgchem.3c01976
Understanding Reactive Sulfur Species through P/S Synergy
Abstract
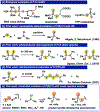
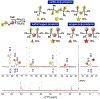
We investigated the differential oxidative and nucleophilic chemistry of reactive sulfur and oxygen anions (SSNO-, SNO-, NO2-, S42-, and HS-) using the simple reducing electrophile PPh2Cl. In the case of SSNO- reacting with PPh2Cl, a complex mixture of mono and diphosphorus products is formed exclusively in the P(V) oxidation state. We found that the phosphine stoichiometry dictates selectivity for oxidation to P=S/P=O products or transformation to P2 species. Interestingly, only chalcogen atoms are incorporated into the phosphorus products and, instead, nitrogen is released in the form of NO gas. Finally, we demonstrate that more reducing anions (S42- and HS-) also react with PPh2Cl with P=S bond formation as a key reaction driving force.
Conflict of interest statement
Conflicts of Interest
There are no conflicts to declare.
Figures
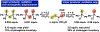

References
-
- Giles GI; Jacob C, Reactive Sulfur Species: An Emerging Concept in Oxidative Stress. Biol. Chem 2002, 383 (3-4), 375–388. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

