MERCURY-3: a randomized comparison of netarsudil/latanoprost and bimatoprost/timolol in open-angle glaucoma and ocular hypertension
- PMID: 37615697
- PMCID: PMC10806046
- DOI: 10.1007/s00417-023-06192-0
MERCURY-3: a randomized comparison of netarsudil/latanoprost and bimatoprost/timolol in open-angle glaucoma and ocular hypertension
Abstract
PURPOSE : To compare the efficacy and safety of the fixed-dose combination (FDC) of netarsudil 0.02%/latanoprost 0.005% ophthalmic solution (NET/LAT; Roclanda®) with bimatoprost 0.03%/timolol maleate 0.5% (BIM/TIM; Ganfort®) ophthalmic solution in the treatment of open-angle glaucoma (OAG) and ocular hypertension (OHT).
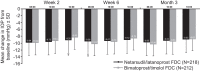
Methods: MERCURY-3 was a 6-month prospective, double-masked, randomized, multicenter, active-controlled, parallel-group, non-inferiority study. Patients (≥ 18 years) with a diagnosis of OAG or OHT in both eyes that was insufficiently controlled with topical medication (IOP ≥ 17 mmHg in ≥ 1 eye and < 28 mmHg in both eyes) were included. Following washout, patients were randomized to once-daily NET/LAT or BIM/TIM for up to 6 months; efficacy was assessed at Week 2, Week 4, and Month 3; safety was evaluated for 6 months. Comparison of NET/LAT relative to BIM/TIM for mean IOP at 08:00, 10:00, and 16:00 h was assessed at Week 2, Week 6, and Month 3. Non-inferiority of NET/LAT to BIM/TIM was defined as a difference of ≤ 1.5 mmHg at all nine time points through Month 3 and ≤ 1.0 mmHg at five or more of nine time points through Month 3.
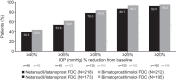
Results: Overall, 430 patients were randomized (NET/LAT, n = 218; BIM/TIM, n = 212), and all received at least one dose of study medication. Efficacy analyses were performed at Month 3 on 388 patients (NET/LAT, n = 184; BIM/TIM, n = 204). NET/LAT demonstrated non-inferiority to BIM/TIM, with a between-treatment difference in IOP of ≤ 1.5 mmHg achieved at all time points and ≤ 1.0 mmHg at the majority of time points (six of nine) through Month 3. Mean diurnal IOP during the study ranged from 15.4 to 15.6 mmHg and 15.2 to 15.6 mmHg in the NET/LAT and BIM/TIM groups respectively, with no between-group statistically significant difference. No significant differences were observed in key secondary endpoints. No serious, treatment-related adverse events (AEs) were observed, and AEs were typically mild/moderate in severity. The most common treatment-related AEs were conjunctival hyperemia (NET/LAT, 30.7%; BIM/TIM, 9.0%) and cornea verticillata (NET/LAT, 11.0%; BIM/TIM, 0%).
Conclusions: Once-daily NET/LAT was non-inferior to BIM/TIM in IOP reduction in OAG and OHT, with AEs consistent with previous findings. NET/LAT offers a compelling alternative FDC treatment option for OAG and OHT.
Keywords: Bimatoprost/timolol; Glaucoma; Intraocular pressure; Netarsudil/latanoprost; Rho-kinase inhibitor.
© 2023. The Author(s).
Conflict of interest statement
IS reports financial support from Aerie Pharmaceuticals Ireland Ltd during the conduct of this study; and grants and personal fees from Théa and Santen, personal fees from Eye-D Pharma, Allergan-Abbvie, Horus and Alcon, and is a co-founder and shareholder of, and consultant for, MONA, outside the submitted work.
KSL reports grants and lecture fees from Santen during the conduct of the study.
FO reports grants and personal fees from Santen and Abbvie outside the submitted work.
MF reports personal fees and non-financial support from Santen outside the submitted work.
JIB reports personal fees from Glaukos, Zeiss and Santen, and personal fees and non-financial support from Esteve, outside the submitted work.
AH reports personal fees from Aerie Pharmaceuticals Ireland Ltd during the conduct of this study, and personal fees from Théa and Santen outside the submitted work.
GL has nothing to disclose.
CS reports personal fees from Abbvie, Horus, Glaukos, Théa, Alcon, Santen, Nicox and Johnson & Johnson outside the submitted work.
BV reports grants from Aerie Pharmaceuticals Ireland Ltd during the conduct of this study and grants from Santen outside the submitted work.
TZ has nothing to disclose.
GH reports personal fees from Santen outside the submitted work.
Figures



References
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

