Involvement of Protein Kinase R in Double-Stranded RNA-Induced Proteasomal Degradation of Hypoxia Inducible Factor-1α
- PMID: 37615898
- PMCID: PMC10673737
- DOI: 10.1007/s10753-023-01881-8
Involvement of Protein Kinase R in Double-Stranded RNA-Induced Proteasomal Degradation of Hypoxia Inducible Factor-1α
Abstract
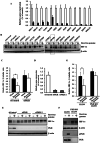
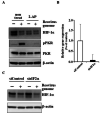
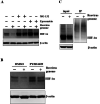
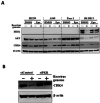
Hypoxia inducible factor-1α (HIF-1α) is a crucial therapeutic target in various diseases, including cancer and fibrosis. We previously demonstrated that transfection with double-stranded RNA (dsRNA), including polyI:C and the dsRNA genome of mammalian orthoreovirus, resulted in significant reduction in HIF-1α protein levels in cultured cells; however, it remained to be elucidated how dsRNA induced down-regulation of HIF-1α protein levels. In this study, we examined the mechanism of dsRNA-mediated down-regulation of HIF-1α protein levels. We found that among the various cellular factors involved in dsRNA-mediated innate immunity, knockdown and knockout of protein kinase R (PKR) significantly restored HIF-1α protein levels in dsRNA-transfected cells, indicating that PKR was involved in dsRNA-mediated down-regulation of HIF-1α. Proteasome inhibitors significantly restored the HIF-1α protein levels in dsRNA-transfected cells. Ubiquitination levels of HIF-1α were increased by transfection with dsRNA. These findings indicated that degradation of HIF-1α in a ubiquitin-proteasome pathway was promoted in a PKR-dependent manner following dsRNA transfection. Expression of not only HIF-1α but also several proteins, including CDK4 and HER2, was down-regulated following dsRNA transfection. These data provide important clues for elucidation of the mechanism of dsRNA-mediated cellular toxicity, as well as for therapeutic application of dsRNA.
Keywords: Double-stranded RNA; HIF-1α; PKR; Proteasome; Ubiquitin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no conflict of interests.
Figures
Similar articles
-
Reovirus double-stranded RNA genomes and polyI:C induce down-regulation of hypoxia-inducible factor 1α.Biochem Biophys Res Commun. 2015 May 15;460(4):1041-6. doi: 10.1016/j.bbrc.2015.03.147. Epub 2015 Apr 2. Biochem Biophys Res Commun. 2015. PMID: 25843794
-
eIF2{alpha} Kinase PKR modulates the hypoxic response by Stat3-dependent transcriptional suppression of HIF-1{alpha}.Cancer Res. 2010 Oct 15;70(20):7820-9. doi: 10.1158/0008-5472.CAN-10-0215. Epub 2010 Oct 5. Cancer Res. 2010. PMID: 20924113
-
TARBP2 Suppresses Ubiquitin-Proteasomal Degradation of HIF-1α in Breast Cancer.Int J Mol Sci. 2021 Dec 24;23(1):208. doi: 10.3390/ijms23010208. Int J Mol Sci. 2021. PMID: 35008634 Free PMC article.
-
Effects of RNA interference-mediated knock-down of hypoxia-inducible factor-α on respiratory burst activity of the Pacific oyster Crassostrea gigas hemocytes.Fish Shellfish Immunol. 2013 Aug;35(2):476-9. doi: 10.1016/j.fsi.2013.05.001. Epub 2013 May 13. Fish Shellfish Immunol. 2013. PMID: 23680843
-
Requirement of hypoxia-inducible factor-1alpha down-regulation in mediating the antitumor activity of the anti-epidermal growth factor receptor monoclonal antibody cetuximab.Mol Cancer Ther. 2008 May;7(5):1207-17. doi: 10.1158/1535-7163.MCT-07-2187. Mol Cancer Ther. 2008. PMID: 18483308
References
-
- Liu, Q., C. Guan, C. Liu, H. Li, W. Jibiao, and C. Sun. 2022. Targeting hypoxia-inducible factor-1alpha: A new strategy for triple-negative breast cancer therapy. Biomedicine and Pharmacotherapy 156. 10.1016/j.biopha.2022.113861. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

