Immune Checkpoint Inhibitors for Child-Pugh Class B Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
- PMID: 37615958
- PMCID: PMC10450588
- DOI: 10.1001/jamaoncol.2023.3284
Immune Checkpoint Inhibitors for Child-Pugh Class B Advanced Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis
Abstract
Importance: Immune checkpoint inhibitors (ICIs) are increasingly used in patients with advanced hepatocellular carcinoma (HCC). However, data on ICI therapy in patients with advanced HCC and impaired liver function are scarce.
Objective: To conduct a systematic review and meta-analysis to determine the efficacy and safety of ICI treatment for advanced HCC with Child-Pugh B liver function.
Data sources: PubMed, Embase, Web of Science, and Cochrane Library were searched for relevant studies from inception through June 15, 2022.
Study selection: Randomized clinical trials, cohort studies, or single-group studies that investigated the efficacy or safety of ICI therapy for Child-Pugh B advanced HCC were included.
Data extraction and synthesis: The Preferred Reporting Items for Systematic Reviews and Meta-Analysis guideline was followed to extract data. A random-effects model was adopted if the heterogeneity was significant (I2 > 50%); otherwise, a fixed-effect model was used.
Main outcomes and measures: The objective response rate (ORR) and overall survival (OS) were considered to be the primary efficacy outcomes of ICI treatment for Child-Pugh B advanced HCC, and the incidence of treatment-related adverse events (trAEs) was set as the primary measure for the safety outcome.
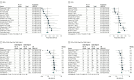
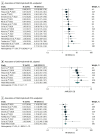
Results: A total of 22 studies including 699 patients with Child-Pugh B and 2114 with Child-Pugh A advanced HCC comprised the analytic sample (median age range, 53-73 years). Upon pooled analysis, patients treated with ICIs in the Child-Pugh B group had an ORR of 14% (95% CI, 11%-17%) and disease control rate (DCR) of 46% (95% CI, 36%-56%), with a median OS of 5.49 (95% CI, 3.57-7.42) months and median progression-free survival of 2.68 (95% CI, 1.85-3.52) months. The rate of any grade trAEs in the Child-Pugh B group was 40% (95% CI, 34%-47%) and of grade 3 or higher trAEs was 12% (95% CI, 6%-23%). Compared with the Child-Pugh A group, the ORR (odds ratio, 0.59; 95% CI, 0.43-0.81; P < .001) and DCR (odds ratio, 0.64; 95% CI, 0.50-0.81; P < .001) were lower in the Child-Pugh B group. Child-Pugh B was independently associated with worse OS in patients with advanced HCC treated with ICIs (hazard ratio, 2.72 [95% CI, 2.34-3.16]; adjusted hazard ratio, 2.33 [95% CI, 1.81-2.99]). However, ICIs were not associated with increased trAEs in the Child-Pugh B group.
Conclusions and relevance: The findings of this systematic review and meta-analysis suggest that although the safety of ICI treatment was comparable between patients with HCC with vs without advanced liver disease and the treatment resulted in a significant number of radiologic responses, survival outcomes are still inferior in patients with worse liver function. More study is needed to determine the effectiveness of ICI treatment in this population.
Conflict of interest statement
Figures

References
-
- Allemani C, Weir HK, Carreira H, et al. ; CONCORD Working Group . Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977-1010. doi: 10.1016/S0140-6736(14)62038-9 - DOI - PMC - PubMed

