Propofol Induced Subacute Cutaneous Lupus Erythematosus: A Case Report
- PMID: 37616181
- PMCID: PMC10467811
- DOI: 10.1213/XAA.0000000000001700
Propofol Induced Subacute Cutaneous Lupus Erythematosus: A Case Report
Abstract
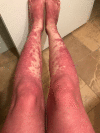
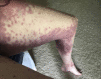
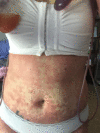
We report a case of a woman who experienced unexplained recurrent rashes of varying severity after multiple exposures to anesthesia, and then 2 successful surgeries under general anesthesia with no resultant rashes after removing propofol from her anesthetic plans. We infer her previous postanesthetic rashes were likely associated with drug-induced subacute cutaneous lupus erythematosus (DI-SCLE) triggered by propofol.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Anesthesia Research Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Folino TB, Muco E, Safadi AO, et al. . Propofol. National Center for Biotechnology Information. Published May 8, 2022. Accessed July 6, 2022. Available at: https://pubmed.ncbi.nlm.nih.gov/28613634/.
-
- DIPRIVAN (propofol) injectable emulsion, USP—Food and Drug Administration. Published April 2017. Accessed March 2, 2022. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019627s066lbl.pdf.
-
- Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. - PubMed
-
- Ben-Menachem E. Systemic lupus erythematosus. Anesth Analg. 2010;111:665–676. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical