Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black Phosphorus Scaffolds for Bone Regeneration
- PMID: 37616380
- PMCID: PMC10558667
- DOI: 10.1002/advs.202302539
Regulation of Osteoimmune Microenvironment and Osteogenesis by 3D-Printed PLAG/black Phosphorus Scaffolds for Bone Regeneration
Abstract
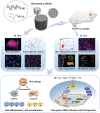
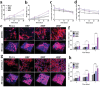
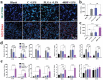
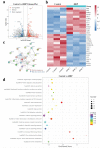
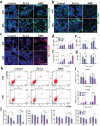
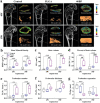
The treatment of bone defects remains a significant challenge to be solved clinically. Immunomodulatory properties of orthopedic biomaterials have significance in regulating osteoimmune microenvironment for osteogenesis. A lactic acid-co-glycolic acid (PLGA) scaffold incorporates black phosphorus (BP) fabricated by 3D printing technology to investigate the effect of BP on osteoimmunomodulation and osteogenesis in site. The PLGA/BP scaffold exhibits suitable biocompatibility, biodegradability, and mechanical properties as an excellent microenvironment to support new bone formation. The studies' result also demonstrate that the PLGA/BP scaffolds are able to recruit and stimulate macrophages M2 polarization, inhibit inflammation, and promote human bone marrow mesenchymal stem cells (hBMSCs) proliferation and differentiation, which in turn promotes bone regeneration in the distal femoral defect region of steroid-associated osteonecrosis (SAON) rat model. Moreover, it is screened and demonstrated that PLGA/BP scaffolds can promote osteogenic differentiation by transcriptomic analysis, and PLGA/BP scaffolds promote osteogenic differentiation and mineralization by activating PI3K-AKT signaling pathway in hBMSC cells. In this study, it is shown that the innovative PLGA/BP scaffolds are extremely effective in stimulating bone regeneration by regulating macrophage M2 polarization and a new strategy for the development of biomaterials that can be used to repair bone defects is offered.
Keywords: 3D-printed Scaffolds; black phosphorus; bone regeneration; macrophage polarization; osteoimmune microenvironment.
© 2023 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
