Collaborative SAR Modeling and Prospective In Vitro Validation of Oxidative Stress Activation in Human HepG2 Cells
- PMID: 37616385
- PMCID: PMC10498489
- DOI: 10.1021/acs.jcim.3c00220
Collaborative SAR Modeling and Prospective In Vitro Validation of Oxidative Stress Activation in Human HepG2 Cells
Abstract
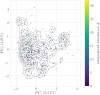
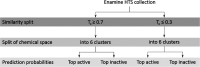
Oxidative stress is the consequence of an abnormal increase of reactive oxygen species (ROS). ROS are generated mainly during the metabolism in both normal and pathological conditions as well as from exposure to xenobiotics. Xenobiotics can, on the one hand, disrupt molecular machinery involved in redox processes and, on the other hand, reduce the effectiveness of the antioxidant activity. Such dysregulation may lead to oxidative damage when combined with oxidative stress overpassing the cell capacity to detoxify ROS. In this work, a green fluorescent protein (GFP)-tagged nuclear factor erythroid 2-related factor 2 (NRF2)-regulated sulfiredoxin reporter (Srxn1-GFP) was used to measure the antioxidant response of HepG2 cells to a large series of drug and drug-like compounds (2230 compounds). These compounds were then classified as positive or negative depending on cellular response and distributed among different modeling groups to establish structure-activity relationship (SAR) models. A selection of models was used to prospectively predict oxidative stress induced by a new set of compounds subsequently experimentally tested to validate the model predictions. Altogether, this exercise exemplifies the different challenges of developing SAR models of a phenotypic cellular readout, model combination, chemical space selection, and results interpretation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Béquignon O. J. M.; Pawar G.; van de Water B.; Cronin M. T.; van Westen G. J.. Systems Medicine; Elsevier, 2021; pp 308–329.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

