Characterizing Phase Transitions of Microfibrillated Cellulose Induced by Anionic and Cationic Surfactants
- PMID: 37616521
- PMCID: PMC10483922
- DOI: 10.1021/acs.langmuir.3c01347
Characterizing Phase Transitions of Microfibrillated Cellulose Induced by Anionic and Cationic Surfactants
Abstract
Rheological modifiers are used to tune rheology or induce phase transitions of products. Microfibrillated cellulose (MFC), a renewable material, has the potential to be used for rheological modification. However, the lack of studies on the evolution in rheological properties and structure during its phase transitions has prevented MFC from being added to consumer, fabric, and home care products. In this work, we characterize surface-oxidized MFC (OMFC), a negatively charged colloidal rod suspension. We measure the rheological properties and structure of OMFC during sol-gel phase transitions induced by either anionic or cationic surfactant using multiple particle tracking microrheology (MPT). MPT tracks the Brownian motion of fluorescent probe particles embedded in a sample, which is related to the sample's rheological properties. Using MPT, we measure that OMFC gelation evolution is dependent on the charge of the surfactant that induces the phase transition. OMFC gelation is gradual in anionic surfactant. In cationic surfactant, gelation is rapid followed by length scale-dependent colloidal fiber rearrangement. Initial OMFC concentration is directly related to how tightly associated the network is at the phase transition, with an increase in concentration resulting in a more tightly associated network with smaller pores. Bulk rheology measures that OMFC forms a stiffer structure but yields at lower strains in cationic surfactant than in anionic surfactant. This study characterizes the role of surfactant in inducing phase transitions, which can be used as a guide for designing future products.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
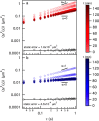
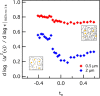
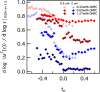

 , calculates the scaling exponents y and z and the critical relaxation exponent n.
, calculates the scaling exponents y and z and the critical relaxation exponent n.



Similar articles
-
Gelation phase diagrams of colloidal rod systems measured over a large composition space.RSC Adv. 2022 Apr 27;12(20):12902-12912. doi: 10.1039/d2ra00609j. eCollection 2022 Apr 22. RSC Adv. 2022. PMID: 35496333 Free PMC article.
-
Combining Microfluidics and Microrheology to Determine Rheological Properties of Soft Matter during Repeated Phase Transitions.J Vis Exp. 2018 Apr 19;(134):57429. doi: 10.3791/57429. J Vis Exp. 2018. PMID: 29733318 Free PMC article.
-
Quantifying the dynamic transition of hydrogenated castor oil gels measured via multiple particle tracking microrheology.Soft Matter. 2016 Aug 14;12(30):6463-72. doi: 10.1039/c6sm00978f. Epub 2016 Jul 11. Soft Matter. 2016. PMID: 27396611
-
A review of nanocrystalline cellulose suspensions: Rheology, liquid crystal ordering and colloidal phase behaviour.Adv Colloid Interface Sci. 2020 Jan;275:102076. doi: 10.1016/j.cis.2019.102076. Epub 2019 Nov 19. Adv Colloid Interface Sci. 2020. PMID: 31780045 Review.
-
Structure and rheology of colloidal particle gels: insight from computer simulation.Adv Colloid Interface Sci. 2013 Nov;199-200:114-27. doi: 10.1016/j.cis.2013.07.002. Epub 2013 Jul 18. Adv Colloid Interface Sci. 2013. PMID: 23916723 Review.
References
-
- Solomon M. J.; Spicer P. T. Microstructural regimes of colloidal rod suspensions, gels, and glasses. Soft Matter 2010, 6, 1391–1400. 10.1039/b918281k. - DOI
-
- De Meirleir N.; Pellens L.; Broeckx W.; De Malsche W. The emulsion crystallization of hydrogenated castor oil into long thin fibers. J. Cryst. Growth 2013, 383, 51–56. 10.1016/j.jcrysgro.2013.08.010. - DOI
-
- Gordon M. B.; Kloxin C. J.; Wagner N. J. The rheology and microstructure of an aging thermoreversible colloidal gel. J. Rheol. 2017, 61, 23–34. 10.1122/1.4966039. - DOI
LinkOut - more resources
Full Text Sources

