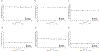A randomized clinical trial to support adherence regimens in children with epilepsy: Examining potential mechanisms of change
- PMID: 37619462
- PMCID: PMC10592186
- DOI: 10.1016/j.yebeh.2023.109393
A randomized clinical trial to support adherence regimens in children with epilepsy: Examining potential mechanisms of change
Abstract
Objective: A family-tailored education and problem-solving intervention, Supporting Treatment Adherence Regimens (STAR), was developed to address the adherence challenges common in youth with epilepsy and their families. Randomized clinical trial (RCT) results indicated a 21% adherence improvement in the STAR group compared with an education-only (EO) group 12-months post-intervention. The current study examined group differences (STAR vs. EO) in epilepsy-specific knowledge, barriers to medication adherence, problem-solving skills, caregiver emotional distress, and family functioning over time and whether these factors mediated group differences in adherence at 12-months post-intervention.
Methods: Two-hundred children (ages 2-12) with epilepsy and their caregivers were included as RCT participants. Children with new-onset epilepsy and adherence <95% were randomized to receive either the STAR (n = 27) or EO (n = 29) intervention. Caregivers completed questionnaires assessing outcomes of interest at baseline, midpoint of the intervention, post-intervention, and 3-, 6-, and 12-month follow-ups. Regression-based analyses of covariance and longitudinal mixed effect linear models were conducted.
Results: Results generally revealed no significant group differences across outcomes of interest at post-intervention or over time. However, one significant model did emerge for social problem-solving skills (b = -1.74, p = 0.04), such that these scores were initially higher in the STAR group compared to the EO group, then decreased slightly in the STAR group over time while remaining stable in the EO group. None of these factors mediated group differences in adherence at 12-months post-intervention.
Conclusion: Future research should examine other potential mechanisms of treatment change after adherence interventions, such as STAR. Nonsignificant findings can inform the development of future study designs and intervention efforts.
Keywords: Adherence; Behavioral intervention; Compliance; Epilepsy; Mediators; Pediatric.
Copyright © 2023 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Avani C. Modi and Shanna Guilfoyle report financial support for this research study was provided by National Institute of Health- Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Figures
References
-
- Faught E, Weiner JR, Guerin A, Cunnington MC, Duh MS. Impact of nonadherence to antiepileptic drugs on health care utilization and costs: findings from the RANSOM study. Epilepsia 2009;50: 501–9. - PubMed
-
- Faught E, Duh MS, Weiner JR, Guerin A, Cunnington MC. Nonadherence to antiepileptic drugs and increased mortality: findings from the RANSOM Study. Neurology 2008;71: 1572–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical