Photodynamic augmentation of oncolytic virus therapy for central nervous system malignancies
- PMID: 37619813
- PMCID: PMC10529118
- DOI: 10.1016/j.canlet.2023.216363
Photodynamic augmentation of oncolytic virus therapy for central nervous system malignancies
Abstract
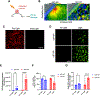
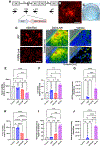
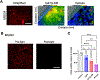
Oncolytic viruses (OVs) have emerged as a clinical therapeutic modality potentially effective for cancers that evade conventional therapies, including central nervous system malignancies. Rationally designed combinatorial strategies can augment the efficacy of OVs by boosting tumor-selective cytotoxicity and modulating the tumor microenvironment (TME). Photodynamic therapy (PDT) of cancer not only mediates direct neoplastic cell death but also primes the TME to sensitize the tumor to secondary therapies, allowing for the combination of two potentially synergistic therapies with broader targets. Here, we created G47Δ-KR, clinical oncolytic herpes simplex virus G47Δ that expresses photosensitizer protein KillerRed (KR). Optical properties and cytotoxic effects of G47Δ-KR infection followed by amber LED illumination (peak wavelength: 585-595 nm) were examined in human glioblastoma (GBM) and malignant meningioma (MM) models in vitro. G47Δ-KR infection of tumor cells mediated KR expression that was activated by LED and produced reactive oxygen species, leading to cell death that was more robust than G47Δ-KR without light. In vivo, we tested photodynamic-oncolytic virus (PD-OV) therapy employing intratumoral injection of G47Δ-KR followed by laser light tumor irradiation (wavelength: 585 nm) in GBM and MM xenografts. PD-OV therapy was feasible in these models and resulted in potent anti-tumor effects that were superior to G47Δ-KR alone (without laser light) or laser light alone. RNA sequencing analysis of post-treatment tumor samples revealed PD-OV therapy-induced increases in TME infiltration of variable immune cell types. This study thus demonstrated the proof-of-concept that G47Δ-KR enables PD-OV therapy for neuro-oncological malignancies and warrants further research to advance potential clinical translation.
Keywords: Glioblastoma; KillerRed; Malignant meningioma; Oncolytic herpes simplex virus; Photodynamic therapy.
Copyright © 2023 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: R.L.M. and S.D.R. are co-inventors on patents relating to oncolytic herpes simplex viruses, owned and managed by Georgetown University and Massachusetts General Hospital (MGH), which have received royalties from Amgen and ActiVec Inc. S.D.R. has received honoraria for expert panel participation from Replimune Inc. All the other authors have no competing interest to declare.
Figures



References
-
- Fudaba H, Wakimoto H, Oncolytic virus therapy for malignant gliomas: entering the new era, Expet Opin. Biol. Ther 23 (2023) 269–282. - PubMed
-
- Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, Batchelor TT, Bindra RS, Chang SM, Chiocca EA, Cloughesy TF, DeGroot JF, Galanis E, Gilbert MR, Hegi ME, Horbinski C, Huang RY, Lassman AB, Le Rhun E, Lim M, Mehta MP, Mellinghoff IK, Minniti G, Nathanson D, Platten M, Preusser M, Roth P, Sanson M, Schiff D, Short SC, Taphoorn MJB, Tonn JC, Tsang J, Verhaak RGW, von Deimling A, Wick W, Zadeh G, Reardon DA, Aldape KD, van den Bent MJ, Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions, Neuro Oncol. 22 (2020) 1073–1113. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

