CALEOSIN 1 interaction with AUTOPHAGY-RELATED PROTEIN 8 facilitates lipid droplet microautophagy in seedlings
- PMID: 37619984
- PMCID: PMC10663143
- DOI: 10.1093/plphys/kiad471
CALEOSIN 1 interaction with AUTOPHAGY-RELATED PROTEIN 8 facilitates lipid droplet microautophagy in seedlings
Abstract
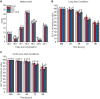
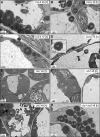
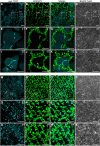
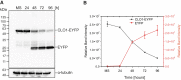
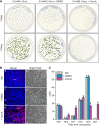
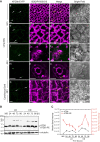
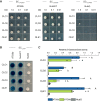
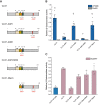
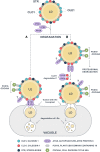
Lipid droplets (LDs) of seed tissues are storage organelles for triacylglycerols (TAGs) that provide the energy and carbon for seedling establishment. In the major route of LD degradation (lipolysis), TAGs are mobilized by lipases. However, LDs may also be degraded via lipophagy, a type of selective autophagy, which mediates LD delivery to vacuoles or lysosomes. The exact mechanisms of LD degradation and the mobilization of their content in plants remain unresolved. Here, we provide evidence that LDs are degraded via a process morphologically resembling microlipophagy in Arabidopsis (Arabidopsis thaliana) seedlings. We observed the entry and presence of LDs in the central vacuole as well as their breakdown. Moreover, we show co-localization of AUTOPHAGY-RELATED PROTEIN 8b (ATG8b) and LDs during seed germination and localization of lipidated ATG8 (ATG8-PE) to the LD fraction. We further demonstrate that structural LD proteins from the caleosin family, CALEOSIN 1 (CLO1), CALEOSIN 2 (CLO2), and CALEOSIN 3 (CLO3), interact with ATG8 proteins and possess putative ATG8-interacting motifs (AIMs). Deletion of the AIM localized directly before the proline knot disrupts the interaction of CLO1 with ATG8b, suggesting a possible role of this region in the interaction between these proteins. Collectively, we provide insights into LD degradation by microlipophagy in germinating seeds with a particular focus on the role of structural LD proteins in this process.
© The Author(s) 2023. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures

References
-
- Akita K, Takagi T, Kobayashi K, Kuchitsu K, Kuroiwa T, Nagata N. Ultrastructural characterization of microlipophagy induced by the interaction of vacuoles and lipid bodies around generative and sperm cells in Arabidopsis pollen. Protoplasma. 2021:258(1):129–138. 10.1007/s00709-020-01557-2 - DOI - PMC - PubMed
-
- Avin-Wittenberg T, Bajdzienko K, Wittenberg G, Alseekh S, Tohge T, Bock R, Giavalisco P, Fernie AR. Global analysis of the role of autophagy in cellular metabolism and energy homeostasis in Arabidopsis seedlings under carbon starvation. Plant Cell. 2015:27(2):306–322. 10.1105/tpc.114.134205 - DOI - PMC - PubMed
-
- Blée E, Boachon B, Burcklen M, Le Guédard M, Hanano A, Heintz D, Ehlting J, Herrfurth C, Feussner I, Bessoule JJ. The reductase activity of the Arabidopsis caleosin RESPONSIVE TO DESSICATION20 mediates gibberellin-dependent flowering time, abscisic acid sensitivity, and tolerance to oxidative stress. Plant Physiol. 2014:166(1):109–124. 10.1104/pp.114.245316 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

