Methylation across the central dogma in health and diseases: new therapeutic strategies
- PMID: 37620312
- PMCID: PMC10449936
- DOI: 10.1038/s41392-023-01528-y
Methylation across the central dogma in health and diseases: new therapeutic strategies
Abstract
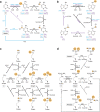
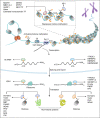
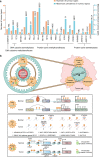
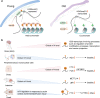
The proper transfer of genetic information from DNA to RNA to protein is essential for cell-fate control, development, and health. Methylation of DNA, RNAs, histones, and non-histone proteins is a reversible post-synthesis modification that finetunes gene expression and function in diverse physiological processes. Aberrant methylation caused by genetic mutations or environmental stimuli promotes various diseases and accelerates aging, necessitating the development of therapies to correct the disease-driver methylation imbalance. In this Review, we summarize the operating system of methylation across the central dogma, which includes writers, erasers, readers, and reader-independent outputs. We then discuss how dysregulation of the system contributes to neurological disorders, cancer, and aging. Current small-molecule compounds that target the modifiers show modest success in certain cancers. The methylome-wide action and lack of specificity lead to undesirable biological effects and cytotoxicity, limiting their therapeutic application, especially for diseases with a monogenic cause or different directions of methylation changes. Emerging tools capable of site-specific methylation manipulation hold great promise to solve this dilemma. With the refinement of delivery vehicles, these new tools are well positioned to advance the basic research and clinical translation of the methylation field.
© 2023. West China Hospital, Sichuan University.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Lysine Methylation Regulators Moonlighting outside the Epigenome.Mol Cell. 2019 Sep 19;75(6):1092-1101. doi: 10.1016/j.molcel.2019.08.026. Mol Cell. 2019. PMID: 31539507 Free PMC article. Review.
-
Epigenomics in stress tolerance of plants under the climate change.Mol Biol Rep. 2023 Jul;50(7):6201-6216. doi: 10.1007/s11033-023-08539-6. Epub 2023 Jun 9. Mol Biol Rep. 2023. PMID: 37294468 Review.
-
Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy.Clin Epigenetics. 2020 Oct 14;12(1):147. doi: 10.1186/s13148-020-00941-2. Clin Epigenetics. 2020. PMID: 33054831 Free PMC article. Review.
-
Histone methylation in pancreatic cancer and its clinical implications.World J Gastroenterol. 2021 Sep 28;27(36):6004-6024. doi: 10.3748/wjg.v27.i36.6004. World J Gastroenterol. 2021. PMID: 34629816 Free PMC article. Review.
-
Evolving insights on histone methylome regulation in human acute myeloid leukemia pathogenesis and targeted therapy.Exp Hematol. 2020 Dec;92:19-31. doi: 10.1016/j.exphem.2020.09.189. Epub 2020 Sep 17. Exp Hematol. 2020. PMID: 32950598 Review.
Cited by
-
Epigenomic Echoes-Decoding Genomic and Epigenetic Instability to Distinguish Lung Cancer Types and Predict Relapse.Epigenomes. 2025 Feb 5;9(1):5. doi: 10.3390/epigenomes9010005. Epigenomes. 2025. PMID: 39982247 Free PMC article. Review.
-
NCOR2 represses MHC class I molecule expression to drive metastatic progression of breast cancer.bioRxiv [Preprint]. 2025 Mar 12:2025.03.10.642060. doi: 10.1101/2025.03.10.642060. bioRxiv. 2025. PMID: 40161756 Free PMC article. Preprint.
-
The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration.Int J Mol Sci. 2025 Aug 3;26(15):7498. doi: 10.3390/ijms26157498. Int J Mol Sci. 2025. PMID: 40806624 Free PMC article. Review.
-
Exploring the complexities of epigenetics in multiple sclerosis: A study involving meta-analysis of DNA methylation profiles, epigenetic drift, and rare epivariations.Mult Scler J Exp Transl Clin. 2024 Dec 5;10(4):20552173241296726. doi: 10.1177/20552173241296726. eCollection 2024 Oct-Dec. Mult Scler J Exp Transl Clin. 2024. PMID: 39651333 Free PMC article.
-
Cyproheptadine inhibits in vitro and in vivo lung metastasis and drives metabolic rewiring.Mol Biol Rep. 2024 Nov 10;51(1):1139. doi: 10.1007/s11033-024-10033-6. Mol Biol Rep. 2024. PMID: 39522095 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

