Immune stress suppresses innate immune signaling in preleukemic precursor B-cells to provoke leukemia in predisposed mice
- PMID: 37620322
- PMCID: PMC10449887
- DOI: 10.1038/s41467-023-40961-z
Immune stress suppresses innate immune signaling in preleukemic precursor B-cells to provoke leukemia in predisposed mice
Abstract
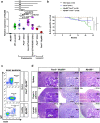
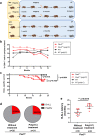
The initial steps of B-cell acute lymphoblastic leukemia (B-ALL) development usually pass unnoticed in children. Several preclinical studies have shown that exposure to immune stressors triggers the transformation of preleukemic B cells to full-blown B-ALL, but how this takes place is still a longstanding and unsolved challenge. Here we show that dysregulation of innate immunity plays a driving role in the clonal evolution of pre-malignant Pax5+/- B-cell precursors toward leukemia. Transcriptional profiling reveals that Myd88 is downregulated in immune-stressed pre-malignant B-cell precursors and in leukemic cells. Genetic reduction of Myd88 expression leads to a significant increase in leukemia incidence in Pax5+/-Myd88+/- mice through an inflammation-dependent mechanism. Early induction of Myd88-independent Toll-like receptor 3 signaling results in a significant delay of leukemia development in Pax5+/- mice. Altogether, these findings identify a role for innate immunity dysregulation in leukemia, with important implications for understanding and therapeutic targeting of the preleukemic state in children.
© 2023. Springer Nature Limited.
Conflict of interest statement
O.B. reports personal fees from Takeda and Clinigen outside the submitted work. K.N. reports grants from Incyte outside the submitted work. No disclosures were reported by the other authors.
Figures




References
-
- Cobaleda C, Vicente-Duenas C, Ramirez-Orellana M, Sanchez-Garcia I. Revisiting the concept of childhood preleukemia. Trends Cancer. 2022;8:887–889. - PubMed
-
- Cobaleda C, Vicente-Duenas C, Sanchez-Garcia I. Infectious triggers and novel therapeutic opportunities in childhood B cell leukaemia. Nat. Rev. Immunol. 2021;21:570–581. - PubMed
-
- Martin-Lorenzo A, et al. Infection exposure is a causal factor in b-cell precursor acute lymphoblastic leukemia as a result of Pax5-inherited susceptibility. Cancer Discov. 2015;5:1328–1343. - PubMed
-
- Rodriguez-Hernandez G, et al. Infection exposure promotes ETV6-RUNX1 precursor B-cell leukemia via impaired H3K4 demethylases. Cancer Res. 2017;77:4365–4377. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

