Consequences of gaining an extra chromosome
- PMID: 37620607
- PMCID: PMC10449985
- DOI: 10.1007/s10577-023-09732-w
Consequences of gaining an extra chromosome
Abstract
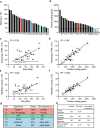
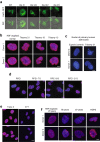
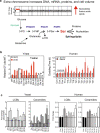
Mistakes in chromosome segregation leading to aneuploidy are the primary cause of miscarriages in humans. Excluding sex chromosomes, viable aneuploidies in humans include trisomies of chromosomes 21, 18, or 13, which cause Down, Edwards, or Patau syndromes, respectively. While individuals with trisomy 18 or 13 die soon after birth, people with Down syndrome live to adulthood but have intellectual disabilities and are prone to multiple diseases. At the cellular level, mistakes in the segregation of a single chromosome leading to a cell losing a chromosome are lethal. In contrast, the cell that gains a chromosome can survive. Several studies support the hypothesis that gaining an extra copy of a chromosome causes gene-specific phenotypes and phenotypes independent of the identity of the genes encoded within that chromosome. The latter, referred to as aneuploidy-associated phenotypes, are the focus of this review. Among the conserved aneuploidy-associated phenotypes observed in yeast and human cells are lower viability, increased gene expression, increased protein synthesis and turnover, abnormal nuclear morphology, and altered metabolism. Notably, abnormal nuclear morphology of aneuploid cells is associated with increased metabolic demand for de novo synthesis of sphingolipids. These findings reveal important insights into the possible pathological role of aneuploidy in Down syndrome. Despite the adverse effects on cell physiology, aneuploidy is a hallmark of cancer cells. Understanding how aneuploidy affects cell physiology can reveal insights into the selective pressure that aneuploid cancer cells must overcome to support unlimited proliferation.
Keywords: Aneuploidy; Down syndrome; Human trisomy; Nuclear morphology; Serine synthesis; Sphingolipids; Yeast.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Agustinus AS, Al-Rawi D, Dameracharla B, Raviram R, Jones B, Stransky S, Scipioni L, Luebeck J, Di Bona M, Norkunaite D, Myers RM, Duran M, Choi S, Weigelt B, Yomtoubian S, McPherson A, Toufektchan E, Keuper K, Mischel PS, Mittal V, Shah SP, Maciejowski J, Storchova Z, Gratton E, Ly P, Landau D, Bakhoum MF, Koche RP, Sidoli S, Bafna V, David Y, Bakhoum SF. Epigenetic dysregulation from chromosomal transit in micronuclei. Nature. 2023;619(7968):176–183. doi: 10.1038/s41586-023-06084-7. - DOI - PMC - PubMed
-
- Azizi E, Carr AJ, Plitas G, Cornish AE, Konopacki C, Prabhakaran S, Nainys J, Wu K, Kiseliovas V, Setty M, Choi K, Fromme RM, Dao P, McKenney PT, Wasti RC, Kadaveru K, Mazutis L, Rudensky AY, Pe'er D. Single-cell map of diverse immune phenotypes in the breast tumor microenvironment. Cell. 2018;174(5):1293–1308 e1236. doi: 10.1016/j.cell.2018.05.060. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

