Hyperoside protects against oxidative stress-mediated photoreceptor degeneration: therapeutic potentials for photoreceptor degenerative diseases
- PMID: 37620913
- PMCID: PMC10463396
- DOI: 10.1186/s12967-023-04459-y
Hyperoside protects against oxidative stress-mediated photoreceptor degeneration: therapeutic potentials for photoreceptor degenerative diseases
Abstract
Background: Photoreceptor degeneration underpinned by oxidative stress-mediated mitochondrial dysfunction and cell death leads to progressive and irreversible vision impairment. Drug treatments that protect against photoreceptor degeneration are currently available in the clinical settings. It has been shown that hyperoside, a flavonol glycoside, protects against neuronal loss in part by suppressing oxidative stress and maintaining the functional integrity of mitochondria. However, whether hyperoside protects against photoreceptor degeneration remains unknown.
Methods: To address the pharmacological potentials of hyperoside against oxidative stress-mediated photoreceptor degeneration on molecular, cellular, structural and functional levels, multiple in vitro and in vivo methodologies were employed in the current study, including live-cell imaging, optical coherence tomography, electroretinography, histological/immunohistochemical examinations, transmission electron microscopy, RNA-sequencing and real-time qPCR.
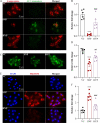
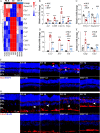
Results: The in vitro results demonstrate that hyperoside suppresses oxidative stress-mediated photoreceptor cell death in part by mitigating mitochondrial dysfunction. The in vivo results reveal that hyperoside protects against photooxidative stress-induced photoreceptor morphological, functional and ultrastructural degeneration. Meanwhile, hyperoside treatment offsets the deleterious impact of photooxidative stress on multiple molecular pathways implicated in the pathogenesis of photoreceptor degeneration. Lastly, hyperoside attenuates photoreceptor degeneration-associated microglial inflammatory activation and reactive Müller cell gliosis.
Conclusions: All things considered, the present study demonstrates for the first time that hyperoside attenuates oxidative stress-induced photoreceptor mitochondrial dysfunction and cell death. The photoreceptor-intrinsic protective effects of hyperoside are corroborated by hyperoside-conferred protection against photooxidative stress-mediated photoreceptor degeneration and perturbation in retinal homeostasis, warranting further evaluation of hyperoside as a photoreceptor protective agent for the treatment of related photoreceptor degenerative diseases.
Keywords: Cell death; Hyperoside; Oxidative stress; Photoreceptor degeneration; Retinal homeostasis.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures








Similar articles
-
Hyperoside mitigates photoreceptor degeneration in part by targeting cGAS and suppressing DNA-induced microglial activation.Acta Neuropathol Commun. 2024 May 16;12(1):76. doi: 10.1186/s40478-024-01793-0. Acta Neuropathol Commun. 2024. PMID: 38755736 Free PMC article.
-
Cuscuta chinensis Lam. Protects Against Light-Induced Retinal Degeneration: Therapeutic Implications for Photoreceptor Degenerative Disorders.Front Pharmacol. 2022 Jun 8;13:904849. doi: 10.3389/fphar.2022.904849. eCollection 2022. Front Pharmacol. 2022. PMID: 35754507 Free PMC article.
-
Astragaloside A Protects Against Photoreceptor Degeneration in Part Through Suppressing Oxidative Stress and DNA Damage-Induced Necroptosis and Inflammation in the Retina.J Inflamm Res. 2022 May 20;15:2995-3020. doi: 10.2147/JIR.S362401. eCollection 2022. J Inflamm Res. 2022. PMID: 35645574 Free PMC article.
-
Unveiling the therapeutic potential and mechanisms of stanniocalcin-1 in retinal degeneration.Surv Ophthalmol. 2025 Jan-Feb;70(1):106-120. doi: 10.1016/j.survophthal.2024.08.001. Epub 2024 Sep 11. Surv Ophthalmol. 2025. PMID: 39270826 Review.
-
The multiple biological activities of hyperoside: from molecular mechanisms to therapeutic perspectives in neoplastic and non-neoplastic diseases.Front Pharmacol. 2025 Mar 3;16:1538601. doi: 10.3389/fphar.2025.1538601. eCollection 2025. Front Pharmacol. 2025. PMID: 40098612 Free PMC article. Review.
Cited by
-
Hyperoside mitigates amphotericin B-induced nephrotoxicity in HK-2 cells via bioenergetic and oxidative stress modulation.BMC Pharmacol Toxicol. 2025 Aug 12;26(1):149. doi: 10.1186/s40360-025-00985-1. BMC Pharmacol Toxicol. 2025. PMID: 40797341 Free PMC article.
-
Hyperoside mitigates photoreceptor degeneration in part by targeting cGAS and suppressing DNA-induced microglial activation.Acta Neuropathol Commun. 2024 May 16;12(1):76. doi: 10.1186/s40478-024-01793-0. Acta Neuropathol Commun. 2024. PMID: 38755736 Free PMC article.
References
-
- Moos WH, Faller DV, Glavas IP, Harpp DN, Kamperi N, Kanara I, Kodukula K, Mavrakis AN, Pernokas J, Pernokas M, et al. Treatment and prevention of pathological mitochondrial dysfunction in retinal degeneration and in photoreceptor injury. Biochem Pharmacol. 2022;203:115168. doi: 10.1016/j.bcp.2022.115168. - DOI - PubMed
-
- Vlachantoni D, Bramall AN, Murphy MP, Taylor RW, Shu X, Tulloch B, Van Veen T, Turnbull DM, McInnes RR, Wright AF. Evidence of severe mitochondrial oxidative stress and a protective effect of low oxygen in mouse models of inherited photoreceptor degeneration. Hum Mol Genet. 2011;20:322–335. doi: 10.1093/hmg/ddq467. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

