α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis
- PMID: 37620916
- PMCID: PMC10463943
- DOI: 10.1186/s40035-023-00372-y
α-Synuclein-carrying astrocytic extracellular vesicles in Parkinson pathogenesis and diagnosis
Abstract
Background: The accumulation of α-synuclein (α-syn), an essential step in PD development and progression, is observed not only in neurons but also in glia, including astrocytes. The mechanisms regulating astrocytic α-syn level and aggregation remain unclear. More recently, it has been demonstrated that a part of α-syn spreading occurs through extracellular vesicles (EVs), although it is unknown whether this process is involved in astrocytes of PD. It is known, however, that EVs derived from the central nervous system exist in the blood and are extensively explored as biomarkers for PD and other neurodegenerative disorders.
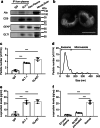
Methods: Primary astrocytes were transfected with A53T α-syn plasmid or exposed to α-syn aggregates. The level of astrocyte-derived EVs (AEVs) was assessed by nanoparticle tracking analysis and immunofluorescence. The lysosomal function was evaluated by Cathepsin assays, immunofluorescence for levels of Lamp1 and Lamp2, and LysoTracker Red staining. The Apogee assays were optimized to measure the GLT-1+ AEVs in clinical cohorts of 106 PD, 47 multiple system atrophy (MSA), and 103 healthy control (HC) to test the potential of plasma AEVs as a biomarker to differentiate PD from other forms of parkinsonism.
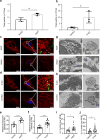
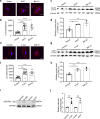
Results: The number of AEVs significantly increased in primary astrocytes with α-syn deposition. The mechanism of increased AEVs was partially attributed to lysosomal dysfunction. The number of α-syn-carrying AEVs was significantly higher in patients with PD than in HC and MSA. The integrative model combining AEVs with total and aggregated α-syn exhibited efficient diagnostic power in differentiating PD from HC with an AUC of 0.915, and from MSA with an AUC of 0.877.
Conclusions: Pathological α-syn deposition could increase the astrocytic secretion of EVs, possibly through α-syn-induced lysosomal dysfunction. The α-syn-containing AEVs in the peripheral blood may be an effective biomarker for clinical diagnosis or differential diagnosis of PD.
Keywords: Astrocyte; Extracellular vesicle; Lysosomal dysfunction; Parkinson’s disease; α-Synuclein.
© 2023. Ruijin Hospital, Shanghai Jiao Tong University.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Niu X, Cheng Y, Hu W, Fan Z, Zhang W, Shao B, et al. Application of bulbocavernosus reflex combined with anal sphincter electromyography in the diagnosis of MSA and PD. Int J Neurosci. 2020:1–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

