Optimizing the fabrication of a 3D high-resolution implant for neural stimulation
- PMID: 37620951
- PMCID: PMC10463680
- DOI: 10.1186/s13036-023-00370-8
Optimizing the fabrication of a 3D high-resolution implant for neural stimulation
Abstract
Background: Tissue-integrated micro-electronic devices for neural stimulation hold great potential in restoring the functionality of degenerated organs, specifically, retinal prostheses, which are aimed at vision restoration. The fabrication process of 3D polymer-metal devices with high resolution and a high aspect-ratio (AR) is very complex and faces many challenges that impair its functionality.
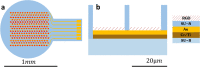
Approach: Here we describe the optimization of the fabrication process of a bio-functionalized 3D high-resolution 1mm circular subretinal implant composed of SU-8 polymer integrated with dense gold microelectrodes (23μm pitch) passivated with 3D micro-well-like structures (20μm diameter, 3μm resolution). The main challenges were overcome by step-by-step planning and optimization while utilizing a two-step bi-layer lift-off process; bio-functionalization was carried out by N2 plasma treatment and the addition of a bio-adhesion molecule.
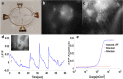
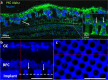
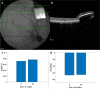
Main results: In-vitro and in-vivo investigations, including SEM and FIB cross section examinations, revealed a good structural design, as well as a good long-term integration of the device in the rat sub-retinal space and cell migration into the wells. Moreover, the feasibility of subretinal neural stimulation using the fabricated device was demonstrated in-vitro by electrical activation of rat's retina.
Conclusions: The reported process and optimization steps described here in detail can aid in designing and fabricating retinal prosthetic devices or similar neural implants.
Keywords: Bio-MEMS; Electrical Neuro-stimulation; Implantable devices; Neural interfaces; Retinal prostheses; SU-8 Photolithography.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
-
- Yue L, Weiland JD, Roska B, Humayun MS. Retinal stimulation strategies to restore vision: Fundamentals and systems Lan. Prog Retin Eye Res. 2016;53:21–47. - PubMed
-
- Charvet G, Rousseau L, Billoint O, Gharbi S, Rostaing J. BioMEA: A versatile high-density 3D microelectrode array system using integrated electronics. Biosens Bioelectron. 2010. 10.1016/j.bios.2010.01.001. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

