PPARγ activation suppresses chondrocyte ferroptosis through mitophagy in osteoarthritis
- PMID: 37620972
- PMCID: PMC10463860
- DOI: 10.1186/s13018-023-04092-x
PPARγ activation suppresses chondrocyte ferroptosis through mitophagy in osteoarthritis
Abstract
Background: Osteoarthritis (OA) is a prevalent disease plaguing the elderly. Recently, chondrocyte ferroptosis has been demonstrated to promote the progression of OA. Peroxisome proliferator-activated receptor-γ (PPARγ) is an important factor in maintaining cartilage health. However, the relationship between PPARγ and chondrocyte ferroptosis in OA and its mechanism is completely unclear.
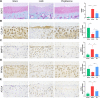
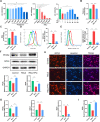
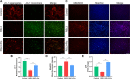
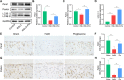
Methods: We established a surgically induced knee OA rat model to investigate PPARγ and chondrocyte ferroptosis in OA. Rat knee specimens were collected for Safranin O/Fast Green staining and immunohistochemical staining after administered orally placebo or pioglitazone (PPARγ agonist) for 4 weeks. We used RSL3 to establish a chondrocyte ferroptosis model cultured in vitro to study the role of PPARγ activation toward ferroptosis, mitochondrial function, and PTEN-induced putative kinase 1 (Pink1)/Parkin-dependent mitophagy. GW9662 (PPARγ antagonist), Mdivi-1 (mitophagy inhibitor), and chloroquine (mitophagy inhibitor) were employed to investigate the mechanism of PPARγ-Pink1/Parkin-dependent mitophagy in the inhibition of ferroptosis.
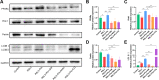
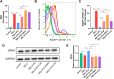
Results: We found that PPARγ activation by pioglitazone attenuated not only OA but also inhibited the expression of the ferroptosis marker acyl-CoA synthetase long-chain family member 4 (ACSL4) at the same time in rats. Furthermore, in vivo and in vitro data indicated that PPARγ activation restored Pink1/Parkin-dependent mitophagy, improved mitochondrial function, inhibited chondrocyte ferroptosis, and delayed the progression of OA.
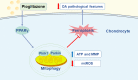
Conclusions: The present study demonstrated that PPARγ activation attenuates OA by inhibiting chondrocyte ferroptosis, and this chondroprotective effect was achieved by promoting the Pink1/Parkin-dependent mitophagy pathway.
Keywords: Chondrocyte; Ferroptosis; Mitophagy; Osteoarthritis; PPARγ; Pink1.
© 2023. BioMed Central Ltd., part of Springer Nature.
Conflict of interest statement
All authors declare that they have no competing interests.
Figures

References
-
- Glyn-Jones S, Palmer AJ, Agricola R, Price AJ, Vincent TL, Weinans H, et al. Osteoarthr Lancet. 2015;386(9991):376–387. - PubMed
-
- van den Berg WB. Osteoarthritis year 2010 in review: pathomechanisms. Osteoarthr Cartil. 2011;19(4):338–341. - PubMed
-
- Chen X, Song QL, Li ZH, Ji R, Wang JY, Cao ML, et al. Pterostilbene ameliorates oxidative damage and ferroptosis in human ovarian granulosa cells by regulating the Nrf2/HO-1 pathway. Arch Biochem Biophys. 2023:109561. - PubMed
-
- Liu H, Zhao Z, Yan M, Zhang Q, Jiang T, Xue J. Calycosin decreases cerebral ischemia/reperfusion injury by suppressing ACSL4-dependent ferroptosis. Arch Biochem Biophys. 2023;734:109488. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

