Sarcopenia assessed by computed tomography or magnetic resonance imaging is associated with the loss of response to biologic therapies in adult patients with Crohn's disease
- PMID: 37621024
- PMCID: PMC10651652
- DOI: 10.1111/cts.13621
Sarcopenia assessed by computed tomography or magnetic resonance imaging is associated with the loss of response to biologic therapies in adult patients with Crohn's disease
Abstract
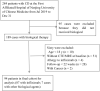
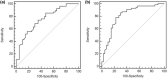
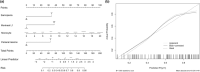
Sarcopenia occurs in patients with Crohn's disease (CD). However, the association between sarcopenia and loss of response (LOR) to biologic agents remains unclear. This study explored such an association in CD patients. This retrospective study included 94 CD patients who received biologic therapy. The skeletal muscle cross-sectional area at the third lumbar was assessed by computed tomography or magnetic resonance imaging for sarcopenia evaluation. A LOR was defined by fecal calprotectin (FC) < 250 μg/g or >50% reduction from baseline levels or other factors, such as the used agent being replaced by other biologic agents. The association between sarcopenia and LOR was assessed by logistic regression analysis. LOR was observed in 54 patients (57.4%). The prevalence of sarcopenia in the LOR group was higher than that in response group (70.4% vs. 40.0%, p = 0.003). Sarcopenia (odds ratio [OR] = 3.89, 95% confidence interval [CI]: 1.31-11.54), Montreal L1 type (OR = 0.20, 95% CI: 0.06-0.60), perianal lesions (OR = 4.08, 95% CI: 1.31-12.70), and monocytes percentage (OR = 1.27, 95% CI: 1.02-1.57) at baseline were independent associated factors for LOR. Sarcopenia was also associated with LOR in patients who received infliximab (OR = 3.31, 95% CI: 1.11-9.87). Montreal L1 type, perianal lesions, and monocytes percentage (Model 1), and with additional consideration of sarcopenia (Model 2), were developed to predict LOR. Model 2 showed better performance than Model 1 (area under the curve [AUC] 0.82 vs. 0.75). Sarcopenia was associated with the LOR to biological agents or infliximab in adult patients with CD.
© 2023 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures
References
-
- Ng SC, Kaplan GG, Tang W, et al. Population density and risk of inflammatory bowel disease: a prospective population‐based study in 13 countries or regions in Asia‐Pacific. Am J Gastroenterol. 2019;114(1):107‐115. - PubMed
-
- Torres J, Mehandru S, Colombel JF, Peyrin‐Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741‐1755. - PubMed
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population‐based studies. Lancet. 2017;390(10114):2769‐2778. - PubMed
-
- Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685‐698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

