Ligand-protected nanocluster-mediated photoswitchable fluorescent nanoprobes towards dual-color cellular imaging
- PMID: 37621438
- PMCID: PMC10445476
- DOI: 10.1039/d3sc03593j
Ligand-protected nanocluster-mediated photoswitchable fluorescent nanoprobes towards dual-color cellular imaging
Abstract
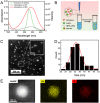
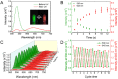
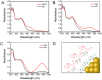
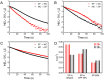
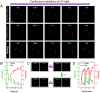
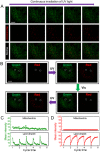
Development of robust multi-color photoswitchable fluorescent probes is critical for many optical applications, but it remains a challenge to rationally design these probes. Here, we report a new design of Förster resonance energy transfer-based dual-color photoswitchable fluorescent nanoparticles (DPF NPs) by taking advantage of the distinct properties of ligand-protected gold nanoclusters (AuNCs). Detailed photophysical studies revealed that ultrasmall-sized AuNCs not only act as the FRET donors due to their intrinsic fluorescence properties, but also play a significant role in regulating the photochromic and aggregate properties of spiropyran through ligand-spiropyran interactions. These DPF NPs exhibit a high fluorescence on/off ratio (∼90%) for both green and red fluorescence emission, and good reversibility during cycled photo-stimulation. Cell imaging experiments showed that DPF NPs could specifically accumulate in lipid droplets, and enable photoswitchable dual-color imaging in living cells. Moreover, by labeling mitochondria with a green-emitting marker, we demonstrated that DPF NPs can distinguish different targets based on dynamic and static fluorescence signals at the sub-cellular level in two emission channels reliably. This study provides a new strategy for designing robust photoswitchable fluorescent probes by modulating the properties of photochromic dyes through ligand-protected nanoclusters, which can be generalized for the development of other photoswitch systems towards advanced optical applications.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Liu Y. Liang S. Yuan C. Best A. Kappl M. Koynov K. Butt H. J. Wu S. Adv. Funct. Mater. 2021;31:2103908. doi: 10.1002/adfm.202103908. - DOI
-
- Imato K. Momota K. Kaneda N. Imae I. Ooyama Y. Chem. Mater. 2022;34:8289–8296. doi: 10.1021/acs.chemmater.2c01809. - DOI
-
- Chai J. Zhao Y. Xu L. Li Q. Hu X. Y. Guo D. S. Liu Y. Angew. Chem., Int. Ed. 2022;61:e202116073. - PubMed
-
- Kim Y. Thangam R. Yoo J. Heo J. Park J. Y. Kang N. Lee S. Yoon J. Mun K. R. Kang M. Min S. Kim S. Y. Son S. Kim J. Hong H. Bae G. Kim K. Lee S. Yang L. Lee J. Y. Kim J. Park S. Kim D. H. Lee K. B. Jang W. Y. Kim B. H. Paulmurugan R. Cho S. W. Song H. C. Kang S. J. Sun W. Zhu Y. Lee J. Kim H. J. Jang H. S. Kim J. S. Khademhosseini A. Kim Y. Kim S. Kang H. Adv. Mater. 2022;34:2205498. doi: 10.1002/adma.202205498. - DOI - PubMed
LinkOut - more resources
Full Text Sources

